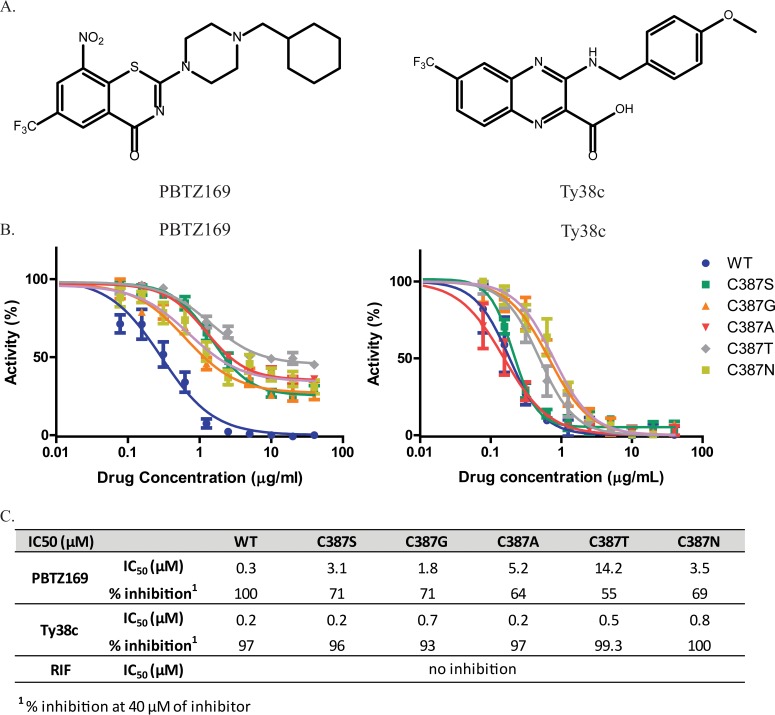
FIG 4.
Effects of DprE1 C387 mutations on potency of DprE1 inhibitor activity. (A) Structures of the DprE1 inhibitors PBTZ169 and Ty38c. PBTZ169 inhibits DprE1 via a covalent bond between the reduced form of its nitro group and the C387 residue of DprE1, whereas Ty38c, which lacks the nitro group, is a noncovalent inhibitor of DprE1. (B) Curves of enzyme activity with increasing inhibitor (PBTZ169 or Ty38c) concentrations for WT and mutant enzymes. A total of 40 μM the inhibitor was used for the highest concentration, with subsequent 2-fold serial dilutions. Enzymatic activities at each inhibitor concentration were normalized to steady-state enzymatic activity in the absence of any inhibitor. (C) PBTZ169 and Ty38c IC50 values and maximum inhibition for WT and mutant enzymes. IC50 values were obtained by fitting the curves in panel B to the log[inhibitor]-versus-normalized response by using GraphPad Prism. Maximum percent inhibition of the WT or mutant enzyme was determined with 40 μM the inhibitor. Data from at least two independent experiments are presented as means ± standard deviations.