Abstract
Candida albicans, normally found as a commensal in the gut, is a major human fungal pathogen responsible for both mucosal and systemic infections in a wide variety of immunocompromised individuals, including cancer patients and organ transplant recipients. The gastrointestinal tract represents a major portal of entry for the establishment of disseminated candidiasis in many of these individuals. Here we report the development of a diet-based mouse model for disseminated candidiasis acquired via the gastrointestinal tract. Using this model, as well as an appropriate immunosuppression regimen, we demonstrate that dissemination of C. albicans from the gastrointestinal tract can result in mortality within 30 days postinfection. We also show a significant increase in fungal burden in systemic organs, but not gastrointestinal tract organs, upon immunosuppression. Importantly, we demonstrate that the administration of two widely used antifungals, fluconazole and caspofungin, either pre- or postimmunosuppression, significantly reduces fungal burdens. This model should prove to be of significant value for testing the ability of both established and experimental therapeutics to inhibit C. albicans dissemination from the gastrointestinal tract in an immunocompromised host as well as the subsequent mortality that can result from disseminated candidiasis.
INTRODUCTION
Candida albicans, the most commonly isolated human fungal pathogen, accounts for about 50% of candidiasis cases and can cause a wide range of both mucosal and life-threatening systemic infections (1, 2). Immunocompromised individuals, including HIV/AIDS patients, recipients of prosthetic devices, cancer patients, and organ transplant recipients, are particularly vulnerable to infection (3–6). About 70% of solid tumor patients develop Candida infections, many of whom have either received chemotherapy and/or undergone surgery in the month prior to infection (7). Candidiasis is also a major cause of death among leukemia patients (7, 8). Approximately $1 billion is spent annually on the treatment of patients with hospital-acquired Candida infections in the United States alone (9).
C. albicans is normally found as a commensal in the gastrointestinal (GI) tract as well as oral and vaginal cavities of healthy individuals (10–12). In immunocompromised patients, the GI tract is a major portal of entry for the establishment of disseminated candidiasis (13–15). However, little is known about the mechanisms that C. albicans uses to invade and translocate across the gastric mucosa as well as the effect of both established and experimental antifungal therapies on these processes. This is largely due to the fact that very few viable animal models are currently available to specifically assess disseminated candidiasis acquired via the GI tract. One previously reported model involves the depletion of normal intestinal flora with antibiotic treatment, colonization with C. albicans, and subsequent administration of the immunosuppressant cyclophosphamide (Cytoxan; Baxter, Deerfield, IL), which leads to complete mortality (16). In this model, dissemination and mortality resulted from the selective depletion of neutrophils along with disruption of the GI mucosa. However, significant increases in liver fungal burden were not observed following the administration of a variety of immunosuppressive regimens. Unlike that of adult mice, the GI tract of neonatal mice has an altered composition of gut immune effectors and antimicrobial peptides and is more amenable to Candida colonization and dissemination (17–21). A major drawback of most neonatal Candida GI models, however, is that colonization and dissemination are transient and rapidly reduced as the mice age (21). GI Candida models involving gnotobiotic, or germfree, mice, including mice with immune deficiencies, have also been reported, although these models require specialized germfree animal facilities, which are not always readily available (21–25).
A number of models have also been established for commensal colonization of the GI tract by C. albicans (16, 26–31). Inoculation is usually performed by oral gavage, and colonization is monitored by determining the fungal burden in fecal pellets at various postinoculation time points. Several of these models have been very useful in the characterization of transcriptional regulators, such as Efh1 and Sfu1, that are involved in controlling the level of C. albicans GI colonization in response to host environmental cues. However, one limitation of these models is that antibiotics, which can have an impact on the level and composition of natural bacterial flora, are used to achieve C. albicans colonization.
In order to address this issue, Yamaguchi et al. described a mouse GI model that uses a purified diet consisting of cornstarch, casein, sucrose, soybean oil, cellulose, l-cystine, choline bitartrate, as well as vitamin and mineral mixes rather than a standard commercial diet (32). The purified diet leads to reduced levels of lactobacilli in the gut as well as a reduction in the organic acids that these bacteria produce. Organic acids, in turn, were shown previously to inhibit the growth of C. albicans. In addition, a previous study suggested that commercial rodent diets contain substances that neutralize fatty acid toxicity against lactobacilli (33), thus raising the levels of these bacteria (and most likely raising organic acid levels in the gut as well). The purified diet thus facilitates C. albicans initial colonization of the GI tract without the added complications of antibiotic use.
In this study, we modify the diet-based approach described above to develop a new mouse model that can be specifically used to examine disseminated candidiasis acquired via the GI tract. We demonstrate that our model can assess both mortality and colonization of the GI tract as well as fungal dissemination to various systemic organs upon immunosuppression. Most importantly, we show that this model can be used to determine the efficacy of multiple antifungals for halting C. albicans dissemination from the GI tract.
MATERIALS AND METHODS
Strain.
C. albicans SC5314 was the strain used in this study, which was previously described (34). We used strain SC5314 because it is the most commonly used wild-type C. albicans strain and the reference strain from which most other C. albicans strains are derived, therefore allowing for a better comparison of our results with those from other studies. In addition, the virulence of this strain has been evaluated in numerous previous studies (11, 35, 36). C. albicans SC5314 was streaked from a glycerol stock at −80°C and grown on yeast extract-peptone-dextrose (YEPD) plates at 30°C for 2 days. Cells were then used to inoculate 50 ml of YEPD liquid medium, and the culture was grown overnight (∼16 to 24 h) at 200 rpm in a 30°C incubator prior to inoculum preparation.
Diet-based mouse GI model of disseminated candidiasis. (i) Dissemination assay.
The model used in this study is based on a model described previously by Yamaguchi et al. (32). Four- to six-week-old male BALB/c mice from Harlan or Charles River Laboratories (weighing 21 to 23 g) were placed on purified rodent diet AIN-93G (catalog number 110700; Dyets, Inc.) for 14 days prior to inoculation. This diet is comprised of α-cornstarch (529.5 g/kg of body weight), casein (200 g/kg), sucrose (100 g/kg), soybean oil (70 g/kg), cellulose (50 g/kg), mineral mix (AIN-93G-MX) (35 g/kg), vitamin mix (AIN-93G-VX) (10 g/kg), l-cystine (3 g/kg), and choline bitartate (2.5 g/kg), as described previously (32, 37). Mice were also placed into wire-bottom cages with paper liners for 24 h so that feces could be collected immediately prior to inoculation (day 0) and every 7 days thereafter until the end of the experiment in order to determine the level of colonization. Food and water were removed from the cages ∼16 and 4 h, respectively, prior to infection. Inoculum preparation and confirmation of inoculum viability were performed as described previously (38) (http://www.sacmm.org/pdf/SOP_MurineModel_GI_Disseminated_Candidiasis_by_Candida_albicans.pdf). Briefly, cells were centrifuged at ∼2,000 rpm for 10 min and washed three times in sterile phosphate-buffered saline (PBS). A loopful (10 μl) of cells was then placed into 2 ml of sterile PBS, spun in a microcentrifuge for 3 min, and resuspended in 2 ml of sterile PBS. Cells were then serially diluted, and the number of cells per milliliter was determined by using a hemocytometer. The inoculum was adjusted to 1.2 × 108 cells/ml in sterile PBS, and 0.5 ml (6.0 × 107 C. albicans cells) was used to inoculate each mouse, similar to the inoculum size described previously by Yamaguchi et al. (32). Inoculum viability was confirmed by carrying out serial dilutions of the inoculum in sterile PBS, plating 100 μl of these dilutions onto Sabouraud dextrose agar, and counting the number of colonies following growth at 37°C overnight. Immunosuppression was carried out by administering cyclophosphamide at 150 mg/kg intraperitoneally (i.p.) and prednisolone sodium succinate (Solu-Delta-Cortef; Pfizer, New York, NY) (32) at 50 mg/kg subcutaneously (s.c.) on days 14 and 17 postinfection. Previous studies have shown that similar cyclophosphamide administration in mice leads to a significant reduction in white blood cell counts and neutropenia (39, 40). In addition, starting on day 14, mice were placed on drinking water with enrofloxacin (Bayer, Shawnee Mission, KS) at 50 ppm (or 50 μg/ml) until the end of the study. Following inoculation, mice were monitored at least twice daily to prevent and minimize unnecessary pain and distress. Moribund animals were euthanized by using 5% isoflurane followed by cervical dislocation. As controls, animals were sacrificed immediately prior to both inoculation (day 0) as well as immunosuppression (day 14). All other mice were sacrificed on day 21. Livers, kidneys, spleens, forestomachs, and small intestines were harvested from all animals, homogenized, and used to assess fungal burden (see http://www.sacmm.org/pdf/SOP_MurineModel_GI_Disseminated_Candidiasis_by_Candida_albicans.pdf). A total of 100 kU/liter penicillin and 100 mg/liter streptomycin were added to the sterile saline used to homogenate the organs in order to prevent bacterial contamination. To evaluate the effects of pre- and postimmunosuppression therapy, antifungals were administered starting 3 days prior to and 4 days following, respectively, the first immunosuppression dose. Commercially available intravenous formulations of fluconazole (Diflucan; Pfizer) and caspofungin (Cancidas; Merck) were administered at 5 mg/kg/day and 1 mg/kg/day, respectively, by i.p. injection.
(ii) Survival assay.
Survival experiments were performed in a manner identical to that for the dissemination experiments described above, using an inoculum of 4.2 × 107 C. albicans cells, with the following exceptions: (i) four doses of the immunosuppression treatment, each consisting of 150 mg/kg cyclophosphamide (i.p.) and 65 mg/kg prednisolone (s.c.), were administered on days 14, 17, 21, and 24 postinfection, and (ii) survival was monitored over the course of 30 days, and all surviving mice were sacrificed on day 30. Fungal burdens in livers, kidneys, spleens, forestomachs, and small intestines were assessed as described above for the dissemination assay.
This animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio, and all animals were maintained in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care.
RESULTS
Establishment of a diet-based mouse model for disseminated candidiasis acquired via the GI tract.
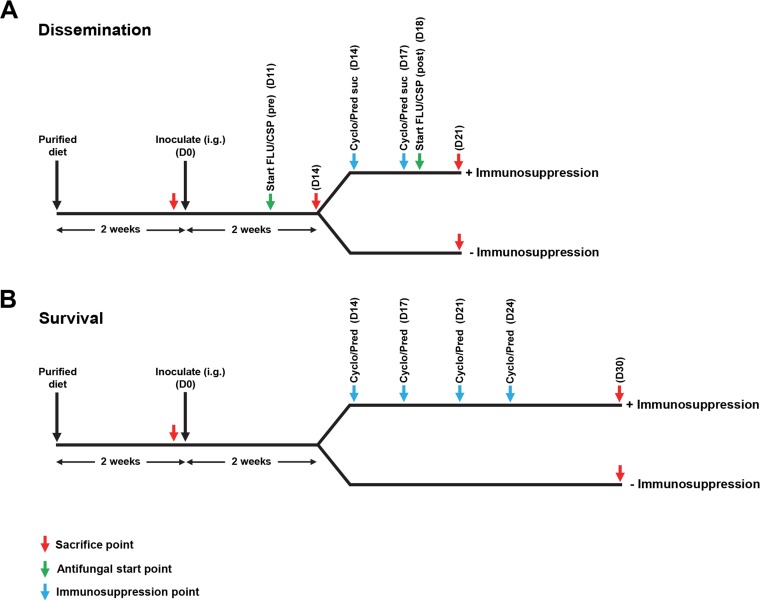
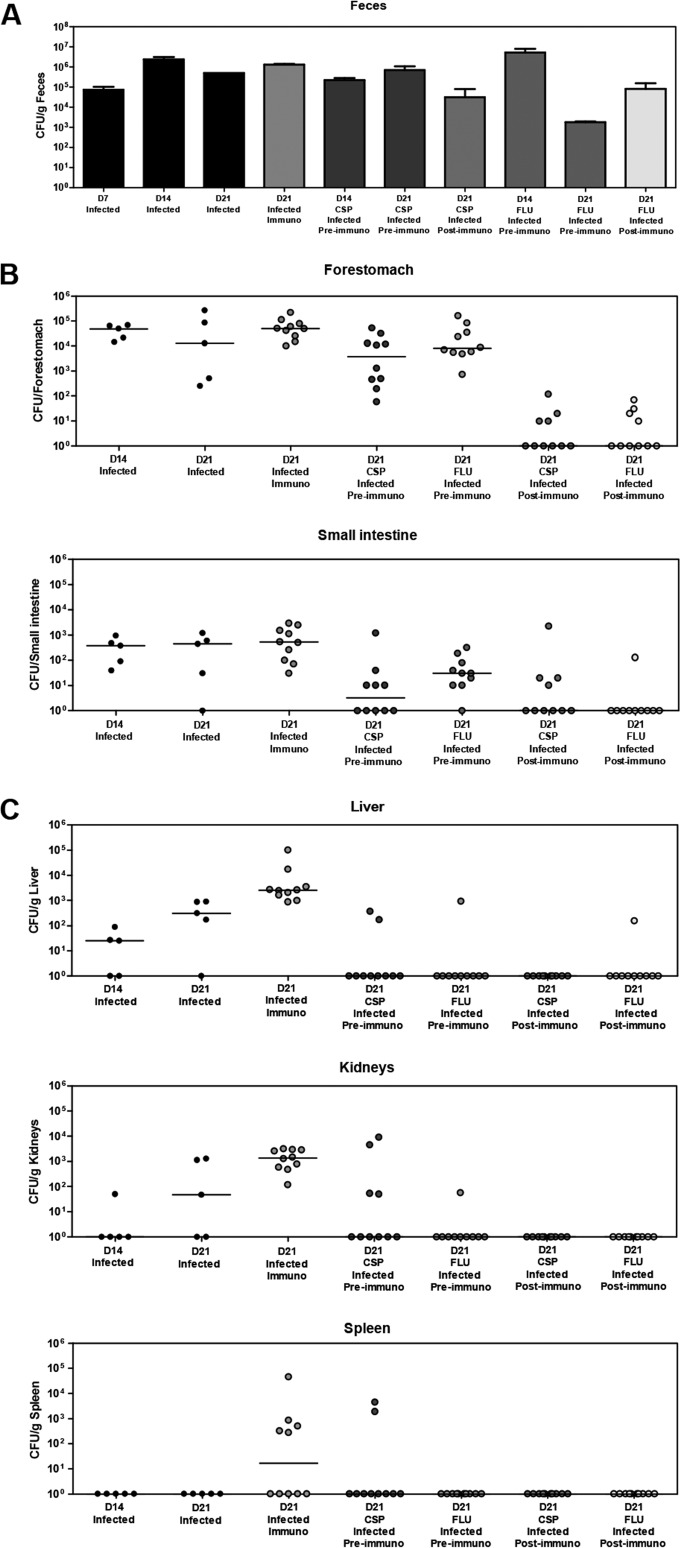
We have developed a diet-based mouse model for GI disseminated candidiasis. The overall experimental strategy for this model is shown in Fig. 1A, and further details are provided in Materials and Methods. Using this model, we observed extensive GI colonization throughout the course of our experiments with fungal burdens generally in the range of 104 to 107 CFU/g feces (Fig. 2A). Initial experiments indicated that GI colonization reached these levels by as early as 3 days postinfection (data not shown). In addition, the level of GI colonization did not appear to be significantly affected by our immunosuppressive treatment. Consistent with these findings, fungal burdens in the GI organs (small intestine and forestomach) also did not appear to show significant differences in immunosuppressed versus control mice (Fig. 2B). In contrast, we observed markedly increased fungal burdens in the systemic organs (livers, kidneys, and spleens) of immunosuppressed versus control animals (Fig. 2C), indicating that immunosuppression was sufficient to promote disseminated infection. Interestingly, while no fungal CFUs were observed in the spleens of nonimmunosuppressed control mice, we still detected fungi in the livers and kidneys of these mice (albeit at lower levels than those observed for the immunosuppressed groups). This observation suggests that there is a low level of background dissemination to these organs that can occur even in the absence of immunosuppression. During the course of optimizing the model described above, we performed experiments using a variety of alternative immunosuppressive regimens. These regimens involved mice receiving a greater number of doses of immunosuppressive treatments prior to sacrifice and/or mice receiving 250 mg/kg cortisone acetate, rather than 50 mg/kg prednisolone sodium succinate. In many of these cases, we found that several mice died prior to the final sacrifice day, complicating our fungal burden analysis. With a large majority of these alternative regimens, we also observed mice dying in our uninfected immunosuppressed control group, indicating that deaths were a consequence of adverse effects/toxicities of the immunosuppressive agents rather than infection.
FIG 1.
Schematic of diet-based models for candidiasis acquired via the gastrointestinal tract. (A) Outline of the model used to assess C. albicans dissemination from the GI tract. Mice were placed on purified diet AIN-93G for 2 weeks and then inoculated by oral gavage (intragastric inoculation [i.g.]) with a wild-type C. albicans strain. After an additional 2 weeks, one group of mice was administered two doses of immunosuppressive treatment, and a second control group was not immunosuppressed. All mice were sacrificed at 21 days postinfection, and both gastrointestinal and systemic organs were harvested for fungal burden analysis. (B) Outline of the model used to assess survival in response to C. albicans gastrointestinal dissemination. A protocol similar to that described above for panel A was followed, except that mice were administered four doses of immunosuppressive treatment and monitored for survival over the course of 30 days. All surviving mice were sacrificed on day 30. FLU, 5 mg/kg fluconazole (i.p.); CSP, 1 mg/kg caspofungin (i.p.); Cyclo, 150 mg/kg cyclophosphamide (i.p.); Pred suc, 50 mg/kg prednisolone sodium succinate (Solu-Delta-Cortef) (s.c.); Pred, 65 mg/kg prednisolone (s.c.); pre, preimmunosuppression; post, postimmunosuppression; D0, day 0.
FIG 2.
Fluconazole and caspofungin inhibit C. albicans dissemination from the GI tract of an immunocompromised host. Mice were infected with 6 × 107 cells of a wild-type C. albicans strain, administered antifungals, immunosuppressed, and sacrificed as described in the legend of Fig. 1A. (A) Mean fungal burdens (± standard errors of the means) in feces of the indicated groups of mice taken at the time points shown. (B) Fungal burdens in gastrointestinal organs from the indicated groups of mice sacrificed at the indicated time points (bars indicate medians) (n = 5 [infected groups on day 14 {D14} or day 21] or n = 10 [all other groups]). (C) Fungal burdens in systemic organs from the indicated groups of mice sacrificed at the indicated time points (bars indicate medians) (n = 5 [infected groups on day 14 or day 21] or n = 10 [all other groups]). No fungal burdens were observed for the uninfected group. CSP, 1 mg/kg caspofungin; FLU, 5 mg/kg fluconazole; Immuno, immunocompromised.
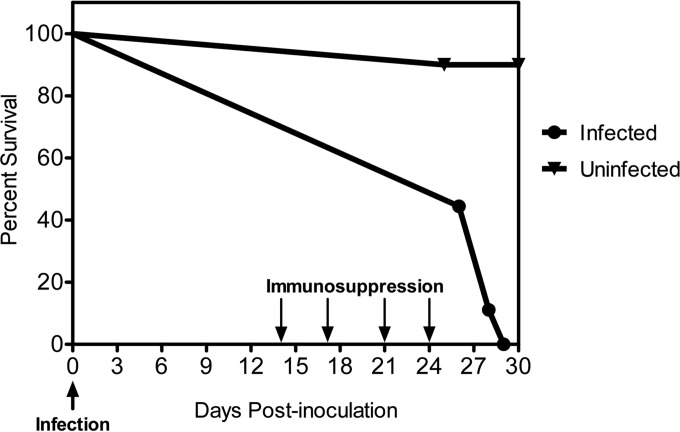
The diet-based model can be used to assess survival specifically in response to disseminated candidiasis acquired via the GI tract.
In order to assess survival in our GI candidiasis model, a more prolonged immunosuppression regimen than that used to test dissemination was required. The experimental strategy for the survival experiment is shown in Fig. 1B, and additional details are provided in Materials and Methods. Briefly, mice were administered four, rather than two, doses of the immunosuppressive treatment and monitored for survival over a course of 30 days. As shown in Fig. 3, about half (five) of the mice in the infected group died by day 26, and all remaining mice in this group were dead by day 29. In contrast, nearly all mice were alive at the end of the experiment (day 30) in an uninfected group that had received the same immunosuppressive regimen, indicating that very few, if any, deaths resulted from the immunosuppression regimen alone. In order to confirm dissemination, both systemic (liver, kidney, and spleen) and GI (small intestine and forestomach) organs were harvested from all animals either at the time of death or at the day 30 sacrifice point, homogenized, and used to determine fungal burdens. Both infected control and infected immunosuppressed groups of mice showed strong GI colonization (see Fig. S1A in the supplemental material) and nearly comparable fungal burdens in the forestomach, although small intestine fungal burdens were somewhat lower in the immunosuppressed group (see Fig. S1B in the supplemental material). As expected, significantly higher fungal burdens were observed in livers, kidneys, and spleens from immunosuppressed mice than in those from nonimmunosuppressed controls (see Fig. S1C in the supplemental material). Overall, these results are consistent with our above-described findings (Fig. 2) and strongly suggest that our model can be used to assess survival in an immunocompromised host specifically in response to disseminated candidiasis acquired via the GI tract.
FIG 3.
C. albicans dissemination from the GI tract reduces survival of an immunocompromised host. Mice were infected with 4.2 × 107 cells of a wild-type C. albicans strain as described in the legend of Fig. 1B. Four consecutive doses of cyclophosphamide (150 mg/kg) and prednisolone (65 mg/kg) were administered to both infected and uninfected control groups at the indicated time points (down arrows), and survival was monitored for 30 days (n = 9 [infected group] or n = 10 [uninfected group]). Based on a log rank analysis, we observed a statistically significant difference between the infected and uninfected groups (P = 0.0002).
Antifungals specifically inhibit C. albicans dissemination from the GI tract.
We next sought to determine the effect of current antifungal therapies on the ability of C. albicans to disseminate from the GI tract using our diet-based model. Our standard assay for dissemination was performed using additional groups of mice that were administered either fluconazole at 5 mg/kg/day i.p. or caspofungin at 1 mg/kg/day i.p. Preimmunosuppression therapy was initiated 3 days prior to immunosuppression, and postimmunosuppression treatment started 4 days following immunosuppression (Fig. 1A). As shown in Fig. 2A, while high levels of GI colonization were generally maintained in the presence and absence of antifungal treatment, several groups showed slight decreases in GI colonization when mice were administered caspofungin or fluconazole either pre- or postimmunosuppression. Preimmunosuppression administration of fluconazole and caspofungin also led to decreases in forestomach and small intestine fungal burdens (Fig. 2B). Most importantly, we observed marked decreases in dissemination to systemic organs (livers, kidneys, and spleens) upon the administration of either caspofungin or fluconazole (Fig. 2C). Reduced dissemination was apparent regardless of whether the antifungals were administered pre- or postimmunosuppression. Overall, our results indicate that administration of fluconazole and caspofungin, either pre- or postimmunosuppression, can significantly halt C. albicans dissemination from the GI tract.
DISCUSSION
Many immunocompromised patients are susceptible and can succumb to C. albicans bloodstream infections that are acquired via the GI tract. As previously indicated, these individuals include cancer patients who are on chemotherapy or have undergone surgery, organ transplant recipients, and neutropenic patients (3–6). In order to most accurately test potential therapeutics that can halt or inhibit the translocation of C. albicans across the gastric mucosa, it is important to develop animal models that most closely emulate physiological and environmental conditions that are encountered in the human host. Because it is difficult to colonize the GI tract of normal healthy rodents with C. albicans, most current models for GI colonization involve the use of antibiotics and, in at least one case, immunosuppression as well (16, 26–31). A limitation of these models, however, is that the administration of antibiotics can influence the level of GI colonization so that it may less accurately simulate that encountered in the host (particularly for hosts who are not receiving antibiotic treatment prior to infection). In addition, immunosuppression is typically used to induce dissemination so that administration of immunosuppressive agents for the purpose of promoting initial colonization makes it difficult for investigators to draw the important distinction between colonization and dissemination.
A major advantage of the mouse GI model for disseminated candidiasis that we have developed in this study is that initial colonization is achieved by using a purified diet rather than antibiotics. The diet-based approach leads to reduced organic acids in the GI tract (compared to levels observed by using commercial diets), which promotes C. albicans growth and reduces the growth of competing bacterial flora (32). Since C. albicans is normally found as a commensal of the mammalian GI tract in healthy individuals, the diet-based approach most likely provides a better approximation of the natural gut environment of humans. Our finding that immunosuppression generally appears to have a greater effect on systemic versus GI organ fungal burdens also strongly suggests that the increases in systemic organ fungal burdens that we observed upon immunosuppression were largely due to an increased translocation of C. albicans across the GI tract (i.e., not an indirect consequence of an increased fungal burden in the GI tract). One limitation of our study, however, is that only a single C. albicans isolate was used. In future studies, it will be interesting to test the accuracy of our model using multiple isolates.
The diet-based C. albicans GI dissemination model that we have developed has a number of important applications. First, the model can be used to test the ability of a wide variety of C. albicans mutants in known virulence processes, such as adhesion, degradative enzyme production, and morphology, to disseminate from the gastric mucosa of an immunocompromised host. These experiments should provide significant insight into specific virulence mechanisms that play an important role in C. albicans GI dissemination. In addition, our demonstration that the model can emulate mortality that occurs specifically as a consequence of C. albicans GI dissemination in an immunosuppressed host opens the avenue for testing the effects of both standard and experimental antifungal therapies on this process. The model can also be used to examine the effect of antifungal treatment on C. albicans dissemination to systemic organs at specific time points following immunosuppression. As a proof of principle, we have already demonstrated that both pre- and postimmunosuppression administration of two standard antifungals, fluconazole and caspofungin, can significantly reduce C. albicans dissemination from the GI tract. Importantly, we observed that both drugs were equally effective when administered either intraperitoneally or by oral gavage (data not shown). These findings strongly suggest that our model could be used to rapidly and effectively test the effects of a variety of experimental antifungal agents on the ability of C. albicans, and potentially additional Candida species as well, to disseminate from the GI tract of an immunocompromised host. Ultimately, results from such experiments are likely to better inform clinicians in designing more effective antifungal regimens for immunocompromised patients at risk of acquiring disseminated candidiasis via the GI tract.
Supplementary Material
ACKNOWLEDGMENTS
We thank Arlene Farias for her assistance with the animal models as well as Mohua Banerjee for technical support. We also thank members of the San Antonio Center for Medical Mycology as well as Scott Filler, Ashraf Ibrahim, and Rory Duncan for their useful advice and suggestions.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01144-16.
REFERENCES
- 1.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarty TP, Pappas PG. 2016. Invasive candidiasis. Infect Dis Clin North Am 30:103–124. doi: 10.1016/j.idc.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Cannon RD, Chaffin WL. 1999. Oral colonization by Candida albicans. Crit Rev Oral Biol Med 10:359–383. doi: 10.1177/10454411990100030701. [DOI] [PubMed] [Google Scholar]
- 4.Dupont PF. 1995. Candida albicans, the opportunist. A cellular and molecular perspective. J Am Podiatr Med Assoc 85:104–115. doi: 10.7547/87507315-85-2-104. [DOI] [PubMed] [Google Scholar]
- 5.Filler SG, Kullberg BJ. 2002. Deep-seated candidal infections, p 341–348. In Calderone R. (ed), Candida and candidiasis. ASM Press, Washington, DC. [Google Scholar]
- 6.Weig M, Gross U, Muhlschlegel F. 1998. Clinical aspects and pathogenesis of Candida infection. Trends Microbiol 6:468–470. doi: 10.1016/S0966-842X(98)01407-3. [DOI] [PubMed] [Google Scholar]
- 7.Viscoli C, Girmenia C, Marinus A, Collette L, Martino P, Vandercam B, Doyen C, Lebeau B, Spence D, Krcmery V, De Pauw B, Meunier F. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin Infect Dis 28:1071–1079. doi: 10.1086/514731. [DOI] [PubMed] [Google Scholar]
- 8.Bow EJ, Loewen R, Cheang MS, Schacter B. 1995. Invasive fungal disease in adults undergoing remission-induction therapy for acute myeloid leukemia: the pathogenetic role of the antileukemic regimen. Clin Infect Dis 21:361–369. doi: 10.1093/clinids/21.2.361. [DOI] [PubMed] [Google Scholar]
- 9.Miller LG, Hajjeh RA, Edwards JE Jr. 2001. Estimating the cost of nosocomial candidemia in the United States. Clin Infect Dis 32:1110. doi: 10.1086/319613. [DOI] [PubMed] [Google Scholar]
- 10.Odds FC. 1994. Pathogenesis of Candida infections. J Am Acad Dermatol 31:S2–S5. doi: 10.1016/S0190-9622(08)81257-1. [DOI] [PubMed] [Google Scholar]
- 11.Odds FC. 1988. Candida and candidosis, 2nd ed Baillière Tindall, London, United Kingdom. [Google Scholar]
- 12.Calderone RA, Clancy CJ (ed). 2012. Candida and candidiasis, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 13.Kullberg BJ, Oude Lashof AM. 2002. Epidemiology of opportunistic invasive mycoses. Eur J Med Res 7:183–191. [PubMed] [Google Scholar]
- 14.Bodey GP. 1997. Disseminated candidiasis in neutropenic patients. Int J Infect Dis 1(Suppl 1):S2–S6. [Google Scholar]
- 15.Myerwitz RL, Pazin GJ, Allen CM. 1977. Disseminated candidiasis. Changes in incidence, underlying diseases and pathology. Am J Clin Pathol 68:29–38. [DOI] [PubMed] [Google Scholar]
- 16.Koh AY, Kohler JR, Coggshall KT, Van Rooijen N, Pier GB. 2008. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog 4:e35. doi: 10.1371/journal.ppat.0040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pope LM, Cole GT, Guentzel MN, Berry LJ. 1979. Systemic and gastrointestinal candidiasis of infant mice after intragastric challenge. Infect Immun 25:702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renz H, Brandtzaeg P, Hornef M. 2012. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol 12:9–23. [DOI] [PubMed] [Google Scholar]
- 19.Kai-Larsen Y, Bergsson G, Gudmundsson GH, Printz G, Jornvall H, Marchini G, Agerberth B. 2007. Antimicrobial components of the neonatal gut affected upon colonization. Pediatr Res 61:530–536. doi: 10.1203/pdr.0b013e318045be83. [DOI] [PubMed] [Google Scholar]
- 20.Domer JE. 1988. Intragastric colonization of infant mice with Candida albicans induces systemic immunity demonstrable upon challenge as adults. J Infect Dis 157:950–958. doi: 10.1093/infdis/157.5.950. [DOI] [PubMed] [Google Scholar]
- 21.Koh AY. 2013. Murine models of Candida gastrointestinal colonization and dissemination. Eukaryot Cell 12:1416–1422. doi: 10.1128/EC.00196-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balish E, Warner TF, Nicholas PJ, Paulling EE, Westwater C, Schofield DA. 2005. Susceptibility of germfree phagocyte oxidase- and nitric oxide synthase 2-deficient mice, defective in the production of reactive metabolites of both oxygen and nitrogen, to mucosal and systemic candidiasis of endogenous origin. Infect Immun 73:1313–1320. doi: 10.1128/IAI.73.3.1313-1320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantorna MT, Balish E. 1990. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect Immun 58:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westwater C, Balish E, Warner TF, Nicholas PJ, Paulling EE, Schofield DA. 2007. Susceptibility of gnotobiotic transgenic mice (Tgepsilon26) with combined deficiencies in natural killer cells and T cells to wild-type and hyphal signalling-defective mutants of Candida albicans. J Med Microbiol 56:1138–1144. doi: 10.1099/jmm.0.47110-0. [DOI] [PubMed] [Google Scholar]
- 25.Westwater C, Schofield DA, Nicholas PJ, Paulling EE, Balish E. 2007. Candida glabrata and Candida albicans; dissimilar tissue tropism and infectivity in a gnotobiotic model of mucosal candidiasis. FEMS Immunol Med Microbiol 51:134–139. doi: 10.1111/j.1574-695X.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- 26.White SJ, Rosenbach A, Lephart P, Nguyen D, Benjamin A, Tzipori S, Whiteway M, Mecsas J, Kumamoto CA. 2007. Self-regulation of Candida albicans population size during GI colonization. PLoS Pathog 3:e184. doi: 10.1371/journal.ppat.0030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C, Pande K, French SD, Tuch BB, Noble SM. 2011. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe 10:118–135. doi: 10.1016/j.chom.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiesner SM, Jechorek RP, Garni RM, Bendel CM, Wells CL. 2001. Gastrointestinal colonization by Candida albicans mutant strains in antibiotic-treated mice. Clin Diagn Lab Immunol 8:192–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekenna O, Sherertz RJ. 1987. Factors affecting colonization and dissemination of Candida albicans from the gastrointestinal tract of mice. Infect Immun 55:1558–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy MJ, Volz PA. 1985. Effect of various antibiotics on gastrointestinal colonization and dissemination by Candida albicans. Sabouraudia 23:265–273. doi: 10.1080/00362178585380391. [DOI] [PubMed] [Google Scholar]
- 31.Mellado E, Cuenca-Estrella M, Regadera J, Gonzalez M, Diaz-Guerra TM, Rodriguez-Tudela JL. 2000. Sustained gastrointestinal colonization and systemic dissemination by Candida albicans, Candida tropicalis and Candida parapsilosis in adult mice. Diagn Microbiol Infect Dis 38:21–28. doi: 10.1016/S0732-8893(00)00165-6. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi N, Sonoyama K, Kikuchi H, Nagura T, Aritsuka T, Kawabata J. 2005. Gastric colonization of Candida albicans differs in mice fed commercial and purified diets. J Nutr 135:109–115. [DOI] [PubMed] [Google Scholar]
- 33.Brockett M, Tannock GW. 1981. Dietary components influence tissue-associated lactobacilli in the mouse stomach. Can J Microbiol 27:452–455. doi: 10.1139/m81-068. [DOI] [PubMed] [Google Scholar]
- 34.Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 35.O'Day DM, Head WS, Csank C, Shetlar DJ, Robinson RD, McCollum GW, Yang R, Zhu TL, Wang MX. 2000. Differences in virulence between two Candida albicans strains in experimental keratitis. Invest Ophthalmol Vis Sci 41:1116–1121. [PubMed] [Google Scholar]
- 36.Luttich A, Brunke S, Hube B, Jacobsen ID. 2013. Serial passaging of Candida albicans in systemic murine infection suggests that the wild type strain SC5314 is well adapted to the murine kidney. PLoS One 8:e64482. doi: 10.1371/journal.pone.0064482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reeves PG, Nielsen FH, Fahey GC Jr. 1993. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951. [DOI] [PubMed] [Google Scholar]
- 38.Najvar LK, Bocanegra R, Wiederhold NP, Lambros C, Najarian N, Patterson TF, Graybill JR. 2008. Therapeutic and prophylactic efficacy of aminocandin (IP960) against disseminated candidiasis in mice. Clin Microbiol Infect 14:595–600. doi: 10.1111/j.1469-0691.2008.01994.x. [DOI] [PubMed] [Google Scholar]
- 39.Zuluaga AF, Salazar BE, Rodriguez CA, Zapata AX, Agudelo M, Vesga O. 2006. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect Dis 6:55. doi: 10.1186/1471-2334-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gudmundsson S, Erlendsdottir H. 1999. Murine thigh infection model, p 137–144. In Zak O, Sande MA (ed), Handbook of animal models of infection. Academic Press, London, United Kingdom. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.