Fig. 10.
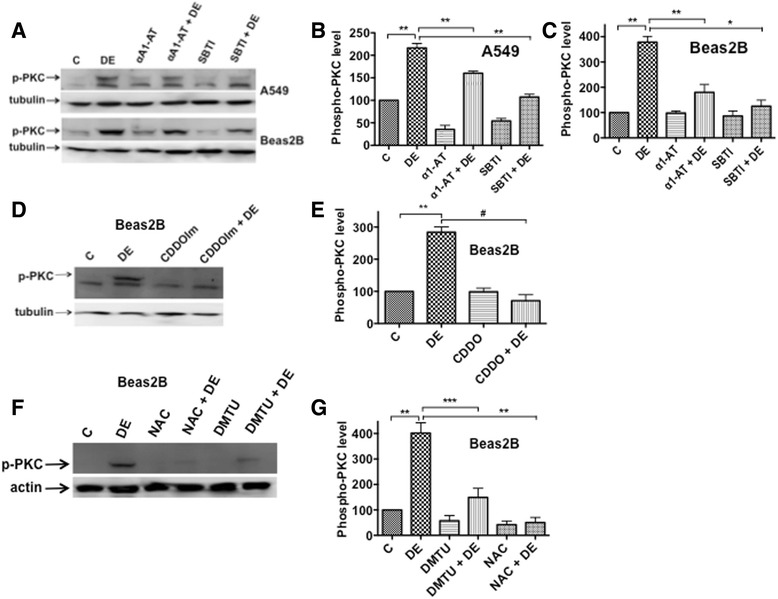
Effects of protease inhibitors and antioxidants on the activation of protein kinase C (PKC). a A549 or Beas2B cells were treated with medium (C), dust extract (1 %) (DE), α1-antitrypsin (25 μg/ml) (α1-AT) or soybean trypsin inhibitor (25 μg/ml) (SBTI) alone, or a combination of DE with α1-AT or SBTI for 5 min. A representative Western blot depicting the levels of phosphorylated PKC and tubulin is shown. b and c Quantification of the effects of α1-AT and SBTI on phosphorylated PKC levels. Levels of phosphorylated PKC and tubulin were quantified and phosphorylated PKC levels normalized to tubulin levels. Levels of phosphorylated PKC in cells treated with medium (C) were arbitrarily considered as 100, and data are shown as means ± SE (n = 3). *P < 0.05; **P < 0.005. d and f Beas2B cells were incubated first with medium (C), 0.5 μM CDDOIm or 15 mM n-acetylcysteine (NAC) adjusted to pH 7.0 for 3 h or 30 mM dimethylthioyrea (DMTU) for 1 h and then treated with or without 1 % dust extract for 5 min. Representative Western blots depicting the levels of phosphorylated PKC, tubulin and actin are shown. e and g Levels of phosphorylated PKC were normalized to tubulin or actin, and levels of phosphorylated PKC in cells treated with medium were arbitrarily considered as 100. Data shown are means ± SE (n = 3). **P < 0.01; ***P < 0.001; #P < 0.0001
