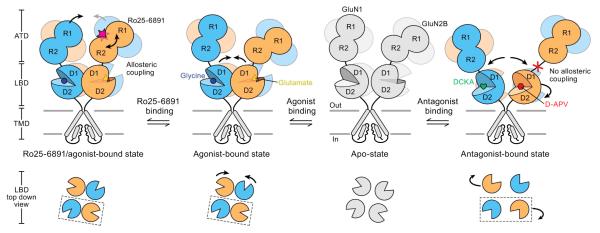
Figure 1. A model of functional interactions between ligand binding domains and amino-terminal domains.
This schematic, based on atomic structures of Xenopus GluN1-GluN2B receptors from Gouaux and colleagues, shows one pair of N1 and N2B subunits of the holoprotein; N1 is in blue and N2B is in orange. Glutamate and glycine binding stabilize the ligand binding domains (LBDs), leading to channel opening. Binding of the ifenprodil derivative Ro25-6891 to the N2 amino-terminal domain (ATD) induces clamshell closure and further affects interactions between the N1 amino-terminal domains. Occupancy and clamshell closure of the amino-terminal domains may increase the ligand binding domain interactions and coupling between the LBD and ATD, consistent with increased receptor activation time course. Competitive antagonist binding (D-APV or DCKA) prevents these allosteric coupling rearrangements. Adapted from Zhu et al., 2016 with permission.