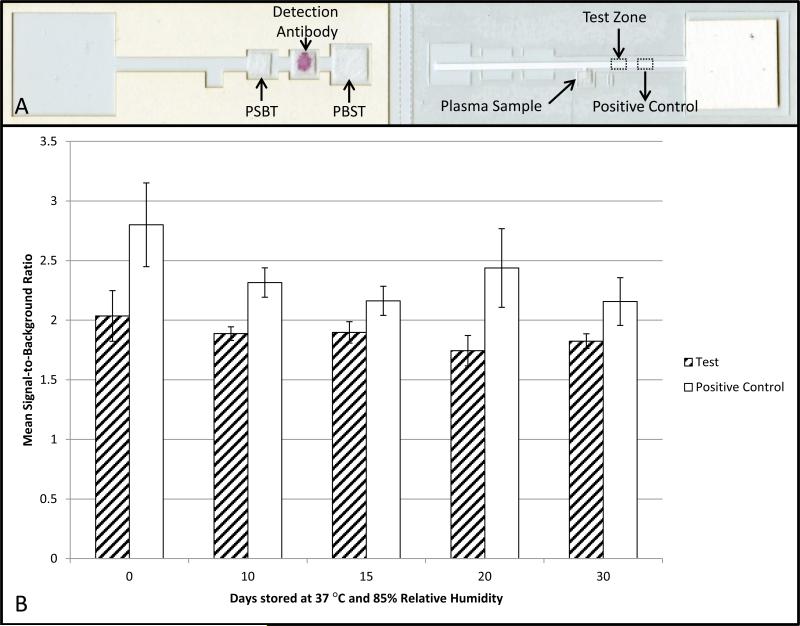
Figure 3. Stability testing of paper-based HPV VLP immunoassays.
Devices were stored at 37°C and 85% relative humidity for 30 days. Each device contained a test zone and positive control zone. The same sample, plasma from an individual who had received 3 HPV vaccines, was used to evaluate the performance of the devices at all time points. The mean signal-to-background ratio decreased 10% over the course of 30 days at the test zone and 23% at the positive control zone.