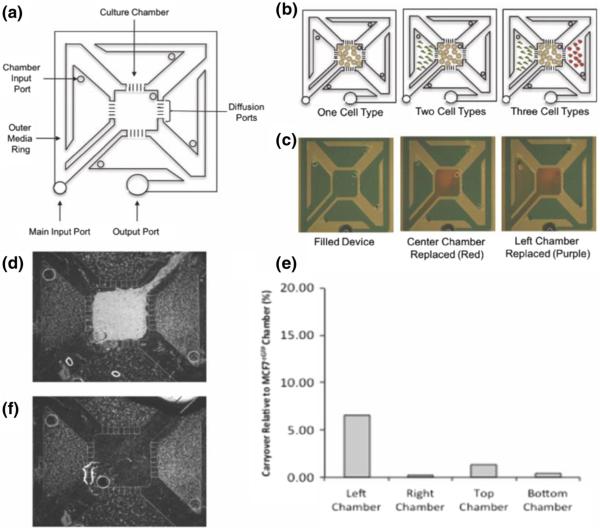
Fig. 1.
Schematic drawing of the compartmentalized micro multi-culture platform and validation of limited cross-contamination. a The micro multiculture device consists of five culture chambers separated by diffusion ports. The device holds a total volume of 50 μL with each culture chamber containing 4 μL. b Cells are seeded into the individual compartments through the chamber input port. Media was changed by replacing the media in the outer ring or in each chamber input port. c Green food grade dye was used to fill the device (50 μL) then 4 μL red dye was pipetted into the center chamber, and 4 μL purple dye was pipetted into the left culture chambers for visualization of addressability of individual compartments. d Fluorescence micrograph of MCF7eGFP cells in the center chamber with HS5 cells in all four side chambers demonstrating maintenance of cell compartmentalization. e Percentage of carryover of cellular lysate in the culture chambers. eGFP mRNA expression levels were measured in HS5 samples after co-culture with MCF7eGFP cells, percentage of carryover over was determined by comparing eGFP expression detected in HS5 cells relative to MCF7eGFP cells at the intermediate time point of 48 h. Between <0.5 % - 6 % cross-contamination was detected, indicating the compartmentalized micro multi-culture device can effectively isolate cellular lysate from the individual culture chambers without the need for downstream separation techniques (n = 2). f Fluorescence micrograph of HS5 cells in the side chambers after MCF7eGFP cells have been selectively removed