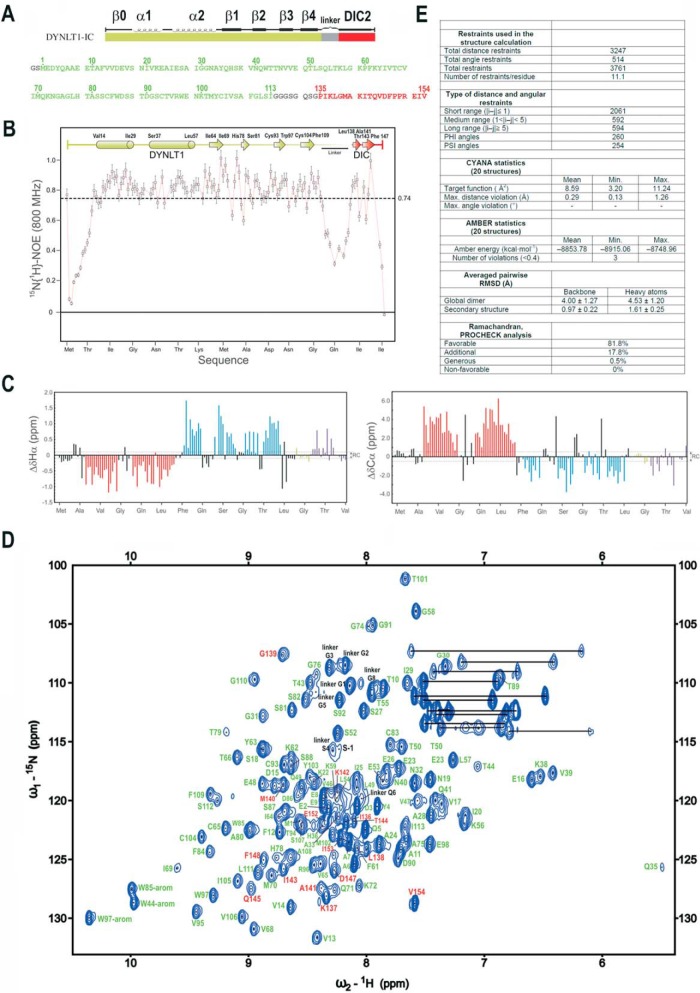
FIGURE 2.
NMR analysis of the DYNLT1-DIC2 chimera. A, design of the chimeric construct in which a linker (in gray) connects DYNLT1 (in green) with a DIC2 fragment (in red). The amino acid sequence is also shown. Numbering corresponds to UniProt P63172 (full-length human DYNLT1, 113 amino acids) and UniProt Q13409 (human DIC2, residues 135–154). B, 1H-15N heteronuclear NOE data of the purified DYNLT1-DIC2 protein. Elements of secondary structure are represented on top. The dotted line represents the average value, which is indicated on the right. C, conformational shift (Δδ) data obtained at 25 °C. Dotted lines represent the limit of the random coil values. D, 1H-15N HSQC spectrum of DYNLT1-DIC2. The amino acid assignment is also shown. Residues corresponding to DYNLT1 are shown in green, whereas those corresponding to the DIC2 part are shown in red. The remaining residues are shown in black as shown in A. E, summary of the statistics corresponding to the NMR calculations. RMSD, root mean square deviation. The error bars indicate 5% of the mean value.