FIGURE 4.
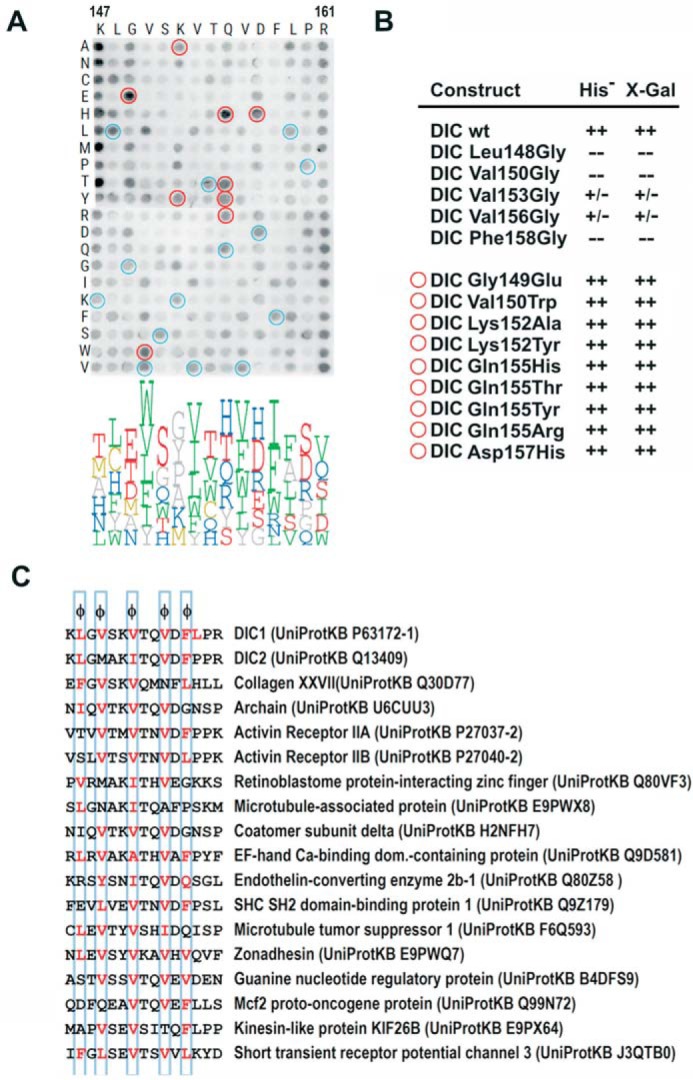
Analysis of the binding plasticity of DYNLT1 canonical groove. A, binding of DYNLT1 to a matrix of pentadecapeptides corresponding to human dynein intermediate chain in which each position was substituted by every natural amino acid. Recombinant DYNLT1 was incubated with a cellulose membrane bearing 300 spots with synthetic pentadecapeptides that expand the binding possibilities. The membrane was developed by ECL after incubation with an anti-DYNLT1 antibody followed by a secondary peroxidase-labeled antibody. A logo distribution is shown at the bottom obtained after densitometric quantification of the spots. Only the six residues with higher signal are shown for clarity. Residues below average signal were not included in the logo representation. Blue circles indicate identical substitutions that serve as positive controls. Red circles indicate substitutions that rendered positive binding and were subsequently confirmed in the yeast two-hybrid assay. B, yeast two-hybrid assay using DYNLT1 in the bait plasmid and various dynein intermediate chain constructs. Growth in the absence of the amino acid His and presence of 3-aminotriazole and X-Gal activity are shown. The upper part shows the binding assay for constructs in which hydrophobic amino acids have been mutated into Gly (L148G, V150G, V153G, V156G, and F158G). The bottom part shows dynein intermediate chain mutants with increased binding to DYNLT1 in the pepscan experiment. In several cases, these mutations comprise non-conservative substitutions and are hence difficult to predict. C, list of proposed cellular DYNLT1-interacting partners according to the results obtained in the pepscan analysis.
