FIGURE 7.
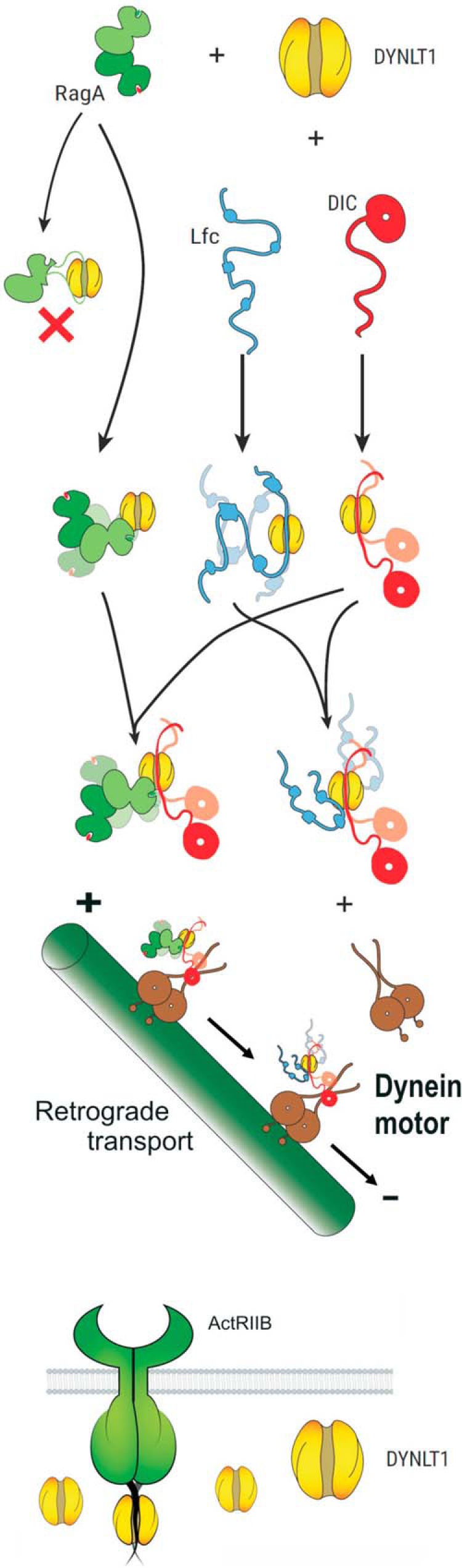
Proposed interaction model of DYNLT1 with various characterized targets. The small GTPases RagA and Rab3D (labeled green) bind to the surface of DYNLT1 and do not occupy the canonical binding groove. Hence, DYNLT1 functions as a dimerization clamp that subsequently links these proteins to DIC (labeled red) and finally to the dynein motor. Conversely, Lfc (labeled in blue) can occupy the canonical binding groove and part of the surface of DYNLT1. Because it is clearly established that DYNLT1 connects Lfc and its homolog GEF-H1 to microtubules (15, 31, 32), binding of DIC would displace Lfc from the binding groove without dissociating the trimeric complex. These guanine nucleotide exchange factors must remain associated to DYNLT1 and become attached to the dynein motor and to microtubules. Finally, ActRIIB and other TGF-β receptors use DYNLT1 (or perhaps its homolog Tctex2β (35)) as molecular clamps to anchor their C termini. It is not known whether DYNLT1 can also mediate heterodimerization of different TGF-β receptors.
