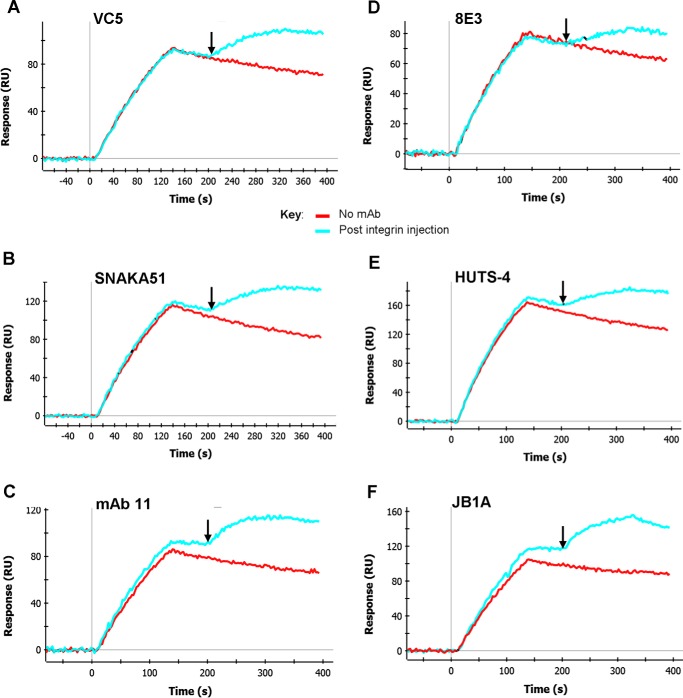
FIGURE 4.
Effect of non-function-blocking anti-α5 and anti-β1 mAbs on integrin-fibronectin complexes. A–F, binding of α5β1-Fc to 50K fibronectin fragment took place for 120 s in three parallel channels in RB. In the blue sensorgrams, integrin was pre-mixed with 100 nm of the indicated anti-α5 mAb (A–C) or anti-β1 mAb (D–F). 60 s after the start of the dissociation phase, at the time indicated by the downward-pointing arrow (∼207 s), either RB alone (red and blue sensorgrams) or RB with 100 nm mAb (cyan sensorgrams) was injected for 120 s. Dissociation rates were measured after the end of the buffer or mAb inject step (330–390 s) for α5β1-fibronectin complexes (red sensorgrams) or mAb-α5β1-fibronectin complexes (cyan sensorgrams). A, dissociation rates were 8.75 × 10−4 and 3.59 × 10−4 s−1, respectively. B, dissociation rates were 11.3 × 10−4 and 3.26 × 10−4 s−1, respectively. C, dissociation rates were 8.47 × 10−4 and 4.47 × 10−4 s−1, respectively. D, dissociation rates were 7.55 × 10−4 and 4.33 × 10−4 s−1, respectively. E, dissociation rates were 8.10 × 10−4 and 3.28 × 10−4 s−1, respectively. F, dissociation rates were 6.73 × 10−4 and 12.9 × 10−4 s−1, respectively. Similar results were obtained in three separate experiments.