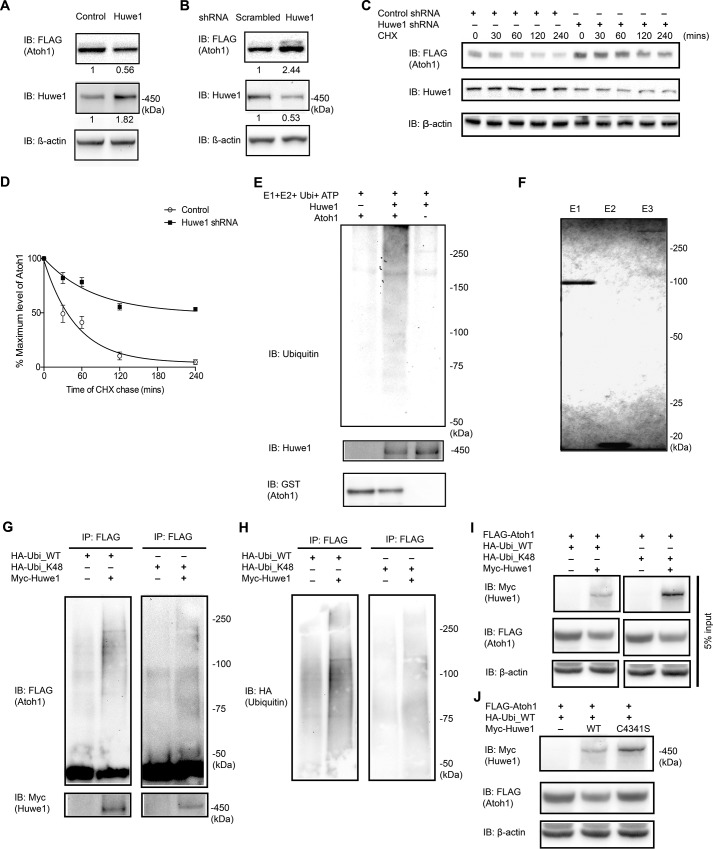
FIGURE 2.
Huwe1 plays a role in the degradation of Atoh1. A, FLAG-HA-Atoh1 293T cells were transfected with Huwe1 (1 μg/ml) for 48 h. Quantification of Atoh1, Huwe1, and loading control (β-actin) was performed by Western blotting (IB) and densitometry (Huwe1 and Atoh1 levels are shown below each lane). B, FLAG-HA-Atoh1 293T cells were transfected with Huwe1 shRNA (100 nm) for 72 h. Quantification of Atoh1 and Huwe1 was performed by Western blotting and densitometry (Huwe1 and Atoh1 levels are shown below each lane) and normalized to a loading control (β-actin). C and D, FLAG-HA-Atoh1 293T cells were transfected with either control or Huwe1 shRNA for 72 h and treated with cycloheximide (CHX, 100 μm) for the indicated times. Atoh1 (FLAG) and Huwe1 were analyzed by Western blotting, and densitometry was normalized to a loading control in three experiments. The ratio of Atoh1 to β-actin was plotted. Error bars indicate S.E. E, recombinant Atoh1 (100 nm) was incubated with ubiquitin, E1 (UBE1), E2 (UbcH5), and full-length Huwe1 in an ATP-regenerating buffer. The reactions were immunoblotted for ubiquitin. F, Coomassie Blue staining of purified E1 (UBE1), E2 (UbcH5), and E3 (Huwe1) proteins used in E. G, HeLa cells were co-transfected with FLAG-Atoh1, HA-ubiquitin (WT) or mutant Lys-48 ubiquitin, and Myc-Huwe1. FLAG-Atoh1 was immunoprecipitated (IP) and subjected to Western blotting with an anti-FLAG antibody for ubiquitylated Atoh1 and an anti-Myc antibody for Huwe1. H, re-blotting of G with an anti-HA (ubiquitin) antibody to show wild-type or Lys-48 polyubiquitin chain conjugated to Atoh1. I, input control for G. J, HeLa cells, co-transfected with FLAG-Atoh1, Myc-Huwe1 (WT) or mutant C4341S Huwe1, and HA-ubiquitin (WT), were lysed and analyzed with an anti-Myc antibody for Huwe1, an anti-FLAG antibody for Atoh1, and an anti-β-actin antibody in the same experiment as shown in I. The mutant Huwe lane is shown with the same Huwe control lanes as in I.