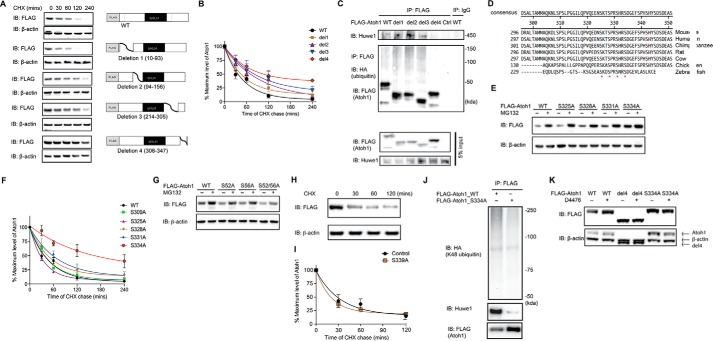
FIGURE 4.
Serine 334 is a critical residue for Atoh1 degradation. A, HEK cells were transfected with either wild-type or a truncated FLAG-Atoh1 (4 mutants as shown) for 48 h and incubated with cycloheximide (CHX; 100 μg/ml) for the indicated times. β-Actin served as a loading control for input protein. B, the ratio of Atoh1 to β-actin based on densitometry was plotted. Data from three experiments are shown. Error bars indicate S.E. C, HEK cells were co-transfected with wild-type or truncated FLAG-Atoh1 and HA-ubiquitin for 48 h. Immunoprecipitation (IP) was performed with anti-FLAG antibody. Ubiquitin and Atoh1 were detected with antibodies to HA and FLAG. Endogenous Huwe1 was detected with an anti-Huwe1 antibody. Immunoprecipitation with IgG was used for the control. Blotting of 5% of total inputs was performed with antibodies against FLAG (Atoh1) and Huwe1. IB, immunoblot. D, conserved serines at 325, 328, 331, and 334 are marked with asterisks in an alignment of the Atoh1 C-terminal region. E, HEK cells were transfected with wild-type or mutated FLAG-Atoh1 for 40 h and treated with either vehicle (DMSO) or proteasome inhibitor (MG132, 10 μm). β-Actin served as a loading control for input protein. F, HEK cells were transfected with wild-type or mutated FLAG-Atoh1 for 40 h and treated with MG132 (10 μm). After 6 h of treatment S334A had the smallest increase in Atoh1 (vehicle treatment is marked with a minus sign) compared with wild-type or other Atoh1 mutants. β-Actin served as a loading control for input protein. The ratio of Atoh1 to β-actin based on densitometry was plotted. Data from three experiments are shown. Error bars indicate S.E. G, HEK cells were transfected with either wild-type or mutated FLAG-Atoh1 for 40 h and incubated with cycloheximide (100 μg/ml) for the indicated times. The ratio of Atoh1 to β-actin based on densitometry was plotted. Data from three experiments are shown. H and I, HEK cells were transfected with either wild-type or S339A FLAG-Atoh1 for 48 h and incubated with cycloheximide (100 μg/ml) for the indicated times. β-Actin served as a loading control (H). The ratio of Atoh1 to β-actin based on densitometry was plotted (I). Data from three experiments are shown. Error bars indicate S.E. J, HEK cells were co-transfected with ubiquitin with all lysines except Lys-48 mutated and wild type (WT) or mutated (S334A) FLAG-Atoh1 for 48 h. Immunoprecipitation was performed with an antibody to FLAG. Huwe1, Atoh1, and ubiquitin were detected with antibodies against Huwe1, HA, and FLAG. K, HEK cells were transfected with wild-type (WT) or mutated FLAG-Atoh1 (deletion 4 (del4) or S334A) for 40 h and treated with either vehicle (DMSO, marked with a minus sign) or D4476 (10 μm). β-Actin served as a loading control for input protein. Additional bands shown in the β-actin panel are from Atoh1 remaining after blot stripping.