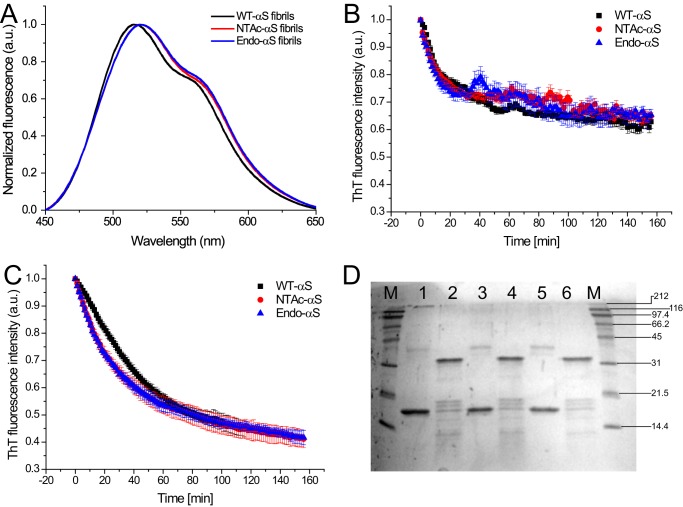
FIGURE 8.
A, fluorescence emission spectra of FE-dye bound to αS fibrils. 20 μm WT-αS (black), NTAc-αS (red), and Endo-αS fibrils (blue) were incubated for 1 h with 2 μm FE-dye in PBS buffer at room temperature. The fluorescence emission spectra were acquired using an excitation wavelength of 420 nm and excitation/emission slit widths at 5 nm. B, stability of αS fibrils to urea exposure followed by ThT fluorescence. Comparable fibril denaturation rates and loss of β-sheet content were observed for WT-αS and the acetylated protein fibrils. C, proteinase K digestion assay wherein the β-sheet content of the fibril solution was followed by ThT fluorescence. The data points in panels B and C represent mean ± S.D. of a minimum 3 independent measurements. D, the corresponding Coomassie-stained SDS-PAGE (12%) gel. Standard molecular weight markers (lane M) are shown on the right side of the gel. Undigested αS fibrils were loaded in lanes 1 (WT-αS), 3 (NTAc-αS), and 5 (Endo-αS), whereas proteinase K-digested fibrils after completion of experiment were loaded in lanes 2 (WT-αS), 4 (NTAc-αS), and 6 (Endo-αS).