FIGURE 6.
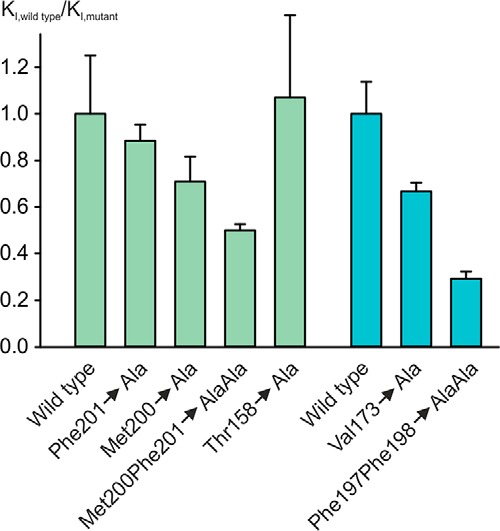
Mutagenesis of hydrophobic binding surface. Affinity of mutants for mono-octanoyltrehalose was measured in binding competition assays. KI values for each mutant are normalized to the KI value for trehalose with that mutant to eliminate the impact of changes on the affinity for trehalose. The only instance in which there is more than a 20% change in the affinity for trehalose is in the case of changing Met-200 to alanine, which increases the KI for trehalose by 3.0 ± 0.1-fold. This effect probably reflects the role of Met-200 in positioning Glu-135, which interacts with glucose residue 2 in the extended sugar-binding site. Results in blue, recalculated from (7), are for the hydrophobic groove, whereas results in green are for the additional hydrophobic surface that interacts with brartemicin. The graph shows the ratio of the normalized KI values for the wild type CRD compared with the mutants, so smaller numbers reflect reduced affinity. Results are reported as the means ± S.D. for n = 3–4 separate experiments, each performed in duplicate.
