FIGURE 1.
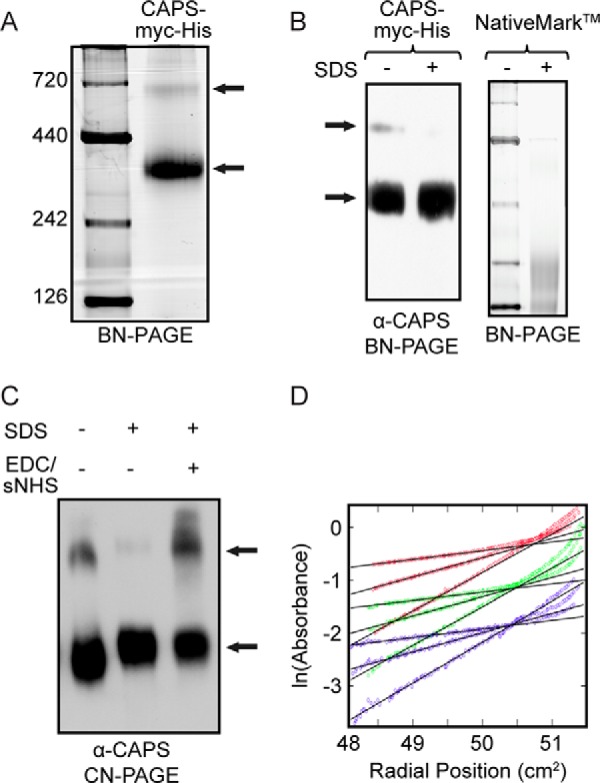
Soluble CAPS is predominantly monomeric. A, BN-PAGE (blue native gel, 6–14%) of NativeMarkTM protein ladder and purified recombinant CAPS-Myc-His, representative of eight similar analyses. B, Western blot (α-CAPS) from BN-PAGE of purified CAPS-Myc-His without or with 2% SDS (left) and NativeMarkTM protein ladder without or with 2% SDS (right), representative of three similar analyses. C, Western blot (α-CAPS) of purified CAPS-Myc-His from CN-PAGE (clear native gel) without or with 2% SDS and with 2% SDS following CAPS cross-linking with 0.5 mm 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and 1.25 mm sulfo-NHS for 2 h at room temperature, representative of two similar studies. Arrows, high mobility CAPS-Myc-His monomer and low mobility CAPS-Myc-His oligomer. D, sedimentation equilibrium data from analytical ultracentrifugation of CAPS. Raw data (squares) are shown for three different speeds (3600 (blue), 6000 (green), and 8800 (red) rpm) with three different loading concentrations for each speed. Fits (black lines) were derived from a non-interconverting two-species model where the observed molecular weight/monomer molecular weight for each species is ∼1.0 and ∼14, corresponding to monomer and higher molecular weight unresolved components, respectively.
