Abstract
Background:
Loss of proprioceptive function occurs after anterior cruciate ligament (ACL) rupture. Clinical, motor, and proprioceptive function is known to improve after ACL reconstruction but does not return to normal. While histological studies of human ACL allografts have been unable to demonstrate mechanoreceptor reinnervation, animal data suggest that reinnervation may occur when an autograft is used.
Purpose:
To compare the presence or absence of mechanoreceptors between allograft versus autograft after ACL reconstruction in humans.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
Ten patients with previous ACL reconstruction presenting for either revision ACL surgery or knee arthroscopy for other reasons were enrolled in a prospective, comparative study. Five patients had a previous autograft ACL and 5 patients had an allograft. Biopsies, either from intact or ruptured grafts, were taken from identical locations as close to the femoral and tibial insertions as possible. Specimens were stained with hematoxylin-eosin (H-E) and monoclonal antibodies against neurofilament protein (NFP), known to be present in mechanoreceptor tissue. Immunohistochemical examination was carried out, and the number of NFP+ neural tissue analogs was counted and compared with that of native ACL tissue.
Results:
The mean time between original graft and biopsy was 6.9 years (range, 0.5-15 years). Histological examination showed significantly less NFP+ neural analogs in allograft and autograft patients than control tissue (mean number of NFP+ analogs per high-power field, 0.7 ± 0.9 [allograft] and 0.5 ± 0.8 [autograft] vs 4.7 ± 0.9 [controls]; P < .0001). There was no significant difference in NFP analogs between autograft and allograft tissue.
Conclusion:
We found a reduced concentration of NFP+ neural analogs in ACL grafts compared with native ACL tissue. This deficit exists irrespective of whether allograft or autograft is used. These findings may explain the continued proprioceptive deficits seen clinically after ACL reconstruction.
Keywords: anterior cruciate ligament, graft, mechanoreceptors, immunohistochemistry
Approximately 200,000 anterior cruciate ligament (ACL) tears occur annually in the United States, and 50% will undergo reconstructive surgery in an attempt to restore stability.22 The functional instability that occurs after ACL rupture is due to both loss of the mechanical restraint to excessive motion and a lack of coordinated muscle activity to compensate and stabilize the knee joint.7 This deficiency in muscular control is thought to be due to loss of proprioceptive feedback from the ACL to the neuromuscular system.4 The human ACL contains mechanoreceptors that detect changes in tension, acceleration, direction of movement, and the position of the knee joint.4,16 Loss of this sensory feedback may impair neuromuscular function and contribute to functional instability after ACL injury.
ACL reconstruction (ACLR) improves clinical, motor, and proprioceptive function compared with the injured state but does not restore it to normal.6,8,10,20,21 Despite restoration of objective measurements of knee laxity, only 45% to 64% of patients return to preinjury levels of sport after ACLR.2,3 Persistent functional impairment may be due to proprioceptive loss, and histological studies of allografts have been unable to demonstrate mechanoreceptor reinnervation after ACLR in humans.13,17 Animal studies suggest reinnervation may occur when autograft is used9; however, human data are lacking. The aim of this study was to compare the presence or absence of mechanoreceptors in allograft versus autograft after ACLR in humans.
Methods
Specimen Harvest
Eleven patients with previous ACLR undergoing either revision ACL surgery or knee arthroscopy for other reasons from September 2012 to February 2013 were enrolled in a prospective, comparative study. Among the 11 specimens, 10 showed acceptable condition and stainability and thus were included in the study. All patients had previously undergone single-bundle ACLR, with no history of any significant posterior cruciate or collateral ligament injury, associated injury/fractures in the knee, or inflammatory arthritis. There were 7 males and 3 females, and the mean age was 33 years (range, 18-45 years).
The mean duration between primary ACL and second-look/revision surgery was 83 months (range, 6-177 months). In revision patients, the elapsed time from reinjury to surgery ranged from 4 weeks to 2 years (Table 1). In 4 revision patients with time from reinjury to specimen harvest >8 weeks, the graft was intact but clinically lax due to fixation failure or tunnel malposition. Five patients had a previous autograft ACL and 5 patients had a previous allograft ACL. As a control, remnant ACL tissue was taken from the tibial and femoral attachment sites of a 33-year-old male 6 weeks after acute primary ACL rupture.
TABLE 1.
Patient Demographicsa
| Patient | Age, y | Sex | Graft Type | Biopsy Type | Time Since Primary ACL Reconstruction, mo | Time From Reinjury to Stump Harvest (Revision Cases), mo |
|---|---|---|---|---|---|---|
| 1 | 37 | M | BTB autograft | Biopsy | 118 | — |
| 2 | 42 | M | BTB autograft | Stump harvest | 177 | 2 |
| 3 | 20 | F | BTB autograft | Stump harvest | 6 | <1 |
| 4 | 46 | M | BTB autograft | Stump harvest | 165 | 24 |
| 5 | 28 | M | Hamstring autograft | Stump harvest | 59 | 11 |
| 6 | 18 | M | BTB allograft | Stump harvest | 6 | <1 |
| 7 | 46 | M | BTB allograft | Biopsy | 130 | — |
| 8 | 24 | M | BTB allograft | Stump harvest | 18 | 10 |
| 9 | 31 | F | BTB allograft | Stump harvest | 11 | 4 |
| 10 | 33 | F | BTB allograft | Biopsy | 140 | — |
| 11 | 33 | M | Native ACL | Stump harvest | 6 weeks postinjury | — |
aACL, anterior cruciate ligament; BTB, bone-tendon-bone; F, female; M, male.
In 7 patients undergoing revision ACLR, all available remnant graft tissue was excised arthroscopically using graspers and a scalpel. The tibial stump was harvested in all cases. In 3 patients with previous ACLR undergoing arthroscopy for other reasons, 2-mm punch biopsies were taken from both the tibial and femoral insertions, as described by Kim et al.17 The harvested specimens were preserved in 10% neutral-buffered formalin solution.
Immunohistochemistry staining was used to identify the presence of neurofilament protein–positive (NFP+) neural tissue analogs in harvested ACL tissue.
ACL Sectioning
Specimens were immersed in optimal cutting temperature (OCT) compound and frozen in preparation for frozen sectioning. The frozen sections were then sequentially sliced at 6-μm thickness, and each slide was placed on a SuperFrost Plus slide (Fisher Scientific) and serially numbered. Multiple sections of each sample were obtained from each specimen (Figure 1). As a single mechanoreceptor can span several 6-μm slices, the specimen was advanced approximately 60 μm between each collected section to avoid overestimating the number of mechanoreceptors. The sections were allowed to dry at room temperature overnight before being fixed in acetone to preserve their antigenicity. Three slides were made for each patient, and each slide had 3 slices that were analyzed for a total of 9 slides per patient.
Figure 1.
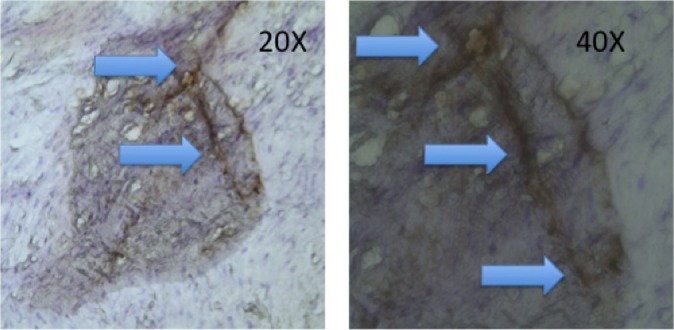
Neurofilament protein–positive (NFP+) neural tissue (arrows) shown at different magnifications in a native anterior cruciate ligament (control) specimen.
Immunohistochemical Staining
Specimens were stained using hematoxylin and monoclonal antibody against NFP. Our procedure of immunohistological staining using the Envision+ HRP System (Dako) was as follows: The primary antibody was anti-NFP-monoclonal mouse antibody (Dako), with a dilution of 1:100. The secondary antibody was peroxidase conjugate. Endogenous peroxidase activity was blocked by adding fully prepared 0.03% hydrogen peroxidase containing sodium azide for placement in a tris-buffered wash bath for 5 minutes. The optimally diluted primary antibody (NFP) was then applied to sections followed by incubation for 30 minutes in a moist chamber. Sections were rinsed gently with buffer solution and placed in a fresh buffer bath. Labeled polymer (peroxidase-labeled polymer conjugated to goat anti-mouse immunoglobulins) was then applied to sections followed by incubation for 30 minutes in a moist chamber. Sections were rinsed gently with buffer solution and placed in a fresh buffer bath. Substrate-chromagen (3-3′-diaminobenzidine chromogen in substrate buffer) was applied to specimens followed by incubation for 7 minutes in a moist chamber. Sections were rinsed gently with distilled water. Counterstaining was done with hematoxylin for 1 minute and followed by mounting with Vectamount (Vector Laboratories).
The specificity of the staining was confirmed by running appropriate control sections with and without the primary monoclonal antibody. All incubation steps were carried out at room temperature in a humidified chamber. Multiple slices from each specimen were serially reviewed.
Interpretation of Histology
All results were interpreted by a single pathologist under a light microscope. The pathologist inspected each slide in a blinded fashion and reviewed the entirety of each section first at 10× and then at 20× magnification for any NFP+ neural tissue. Stained specimens were examined, cells were identified, and the number and location on the slides were marked. Positive results were given to granularly stained areas with NFP (Figure 2). The NFP monoclonal antibody stained axon cylinders carrying proprioceptive fibers, and hematoxylin was used as a counterstain for detailed histological evaluation. All recordings were tracked with a manual cell counter and recorded.
Figure 2.
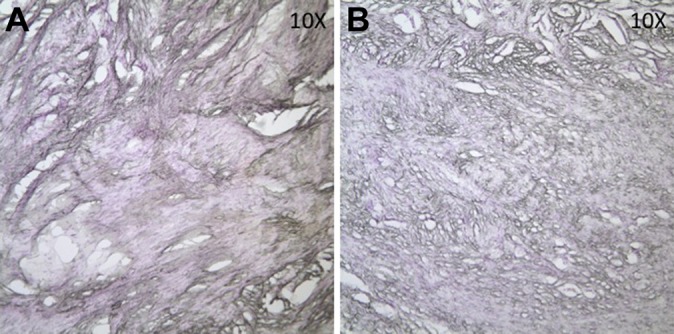
Anterior cruciate ligament graft sections showing absence of neurofilament protein–stained neural tissue. (A) Representative autograft section; (B) representative allograft section.
Statistical Analysis
Data are expressed as the mean ± SD. An unpaired t test (Prism Software; Graphpad Software Inc) was used to compare the number of NFP+ neural tissue analogs per slide between graft and control ACL tissue, with 9 slides analyzed per patient. All statistical tests were 2-tailed, and a P value (2-tailed) <.05 was assigned as significant.
Results
There were NFP+ neural tissue analogs visible in the control patient’s slides (Figure 1) and a reduced concentration of NFP+-stained neural tissue analogs in the graft sections (Figure 2). None of the entire graft specimens had more than 3 NFP+ analogs, while none of the control sections had less than 3 NFP+ analogs.
The mean number of NFP+ neural tissue analogs per slide in the autograft samples was significantly decreased compared with the control samples (0.5 ± 0.78 vs 4.7 ± 0.87, respectively; P < .0001). The number of NFP+ neural tissue analogs per slide in the allograft samples was also significantly decreased compared with the control samples (0.7 ± 0.85 vs 4.7 ± 0.87, respectively; P < .0001). In contrast, there was no statistically significant difference in the number of NFP+ neural tissue analogs in the autograft compared with allograft samples (Figure 3).
Figure 3.
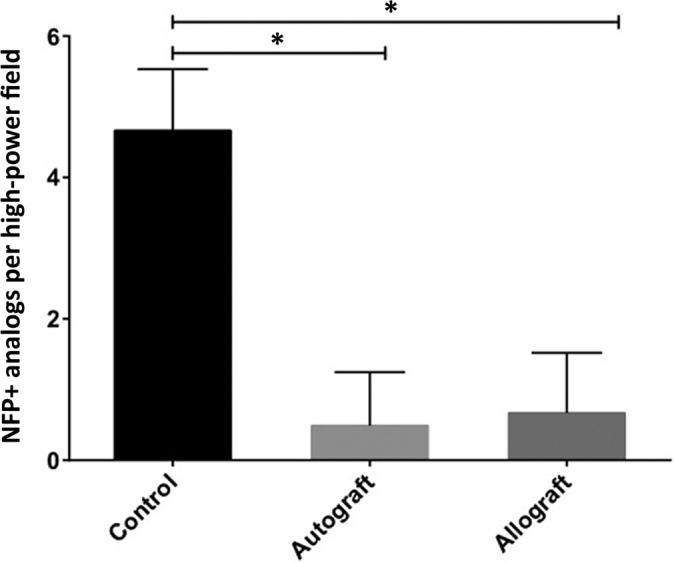
Comparison of number of NFP+ analogs between graft types and controls (control, n = 1; autograft and allograft, n = 5). *P ≤ .0001. NFP+, neurofilament protein–positive.
Discussion
This study found markedly reduced concentrations of NFP+ neural tissue in ACL grafts compared with native ACL tissue. This deficit persisted irrespective of graft choice and time since surgery, suggesting minimal reinnervation of ACL grafts occurs in humans. These findings may explain the continued proprioceptive deficit noted clinically after ACL reconstruction.10
Traditionally, mechanoreceptors have been identified using histological stains such as gold chloride,17 then classified using morphological criteria by Freeman and Wyke.12 However, recent studies have demonstrated that immunohistochemical methods are a more reliable method of identifying mechanoreceptor tissue.4,11 Fromm and Kummer13 reported immunofluorescent stains could be used to group neurons in ACLs into 3 types, each of which can be stained with an antibody. Fast-conducting mechanoreceptive sensory afferent nerve fibers were characterized by the presence of NFP, slow-conducting nociceptive sensory efferent fibers by substance P, and sympathetic postganglionic neurons utilizing noradrenaline as transmitted were identified with antisera directed against the rate-limiting enzyme of noradrenaline synthesis, tyrosine hydroxylase. We chose to use immunohistochemical staining for NFP as our specific interest was mechanoreceptor fibers contributing to proprioception function.
Two previous studies have attempted to identify mechanoreceptor reinnervation of ACL grafts in humans, with conflicting findings. Kim et al17 analyzed tissue biopsies in 11 patients who had undergone allograft ACLR 12 to 120 months previously. They found no histological evidence of mechanoreceptor ingrowth in any biopsies. Denti et al9 analyzed samples from 2 patients with failed ACL hamstring autografts 9 and 10 years postsurgery using gold chloride staining. They reported “a significant number” of Ruffini and Pacini corpuscles, particularly near the tibial insertion. Using modern immunohistochemical techniques, we were able to find evidence suggesting mechanoreceptor reinnervation in both autograft and allograft tissue, although in markedly reduced numbers, compared with our control specimen.
Data from animal studies is similarly mixed. In a rabbit model, Fromm and Kummer13 demonstrated that no mechanoreceptors had reinnervated an ACL allograft by 52 weeks postoperatively using immunofluorescent stains for NFP. In contrast, Denti et al9 reported histological evidence of mechanoreceptors using gold chloride stains in sheep ACL autografts. Barrack et al5 measured somatosensory-evoked potentials (SEPs) in a canine model of ACL autografts and found that only 2 of 6 dogs showed evidence of reinnervation 6 months after ACLR.
SEPs after ACL stimulation have also been reported in humans. Ochi et al20 compared SEPs with intraoperative stimulation between 14 patients with normal ACLs and 22 patients 18 months post–hamstring autograft ACLR. While SEPs were found in all reconstructed patients, voltages were lower than in patients with a native ACL. It should be noted that SEPs do not specifically detect mechanoreceptor function, and this finding together with data from our and other histological studies suggest any reinnervation that occurs after ACLR is incomplete.
A number of studies have analyzed the presence of mechanoreceptors in the tibial remnant after ACL rupture and prior to ACLR. In 2 studies looking at 94 ruptured ACL remnants, Bali et al4 and Dhillon et al11 found that mechanoreceptors were more common at insertion sites of the ACL, which was the rationale for taking biopsies at insertion sites in our study. They also found that NFP-containing tissue was increased with shorter duration from injury, longer stumps, and stumps that showed adherence to the posterior cruciate ligament (PCL). This suggests that the number of mechanoreceptors declines after ACL rupture, and these 3 factors slow or arrest the rate of decline. Similarly, Georgoulis et al14 analyzed 17 ACL remnants using gold chloride staining and found that mechanoreceptors were more likely to be present if the ACL remnant had healed down to the PCL. This suggests that continued stretch and stimulation may be important to the maintenance of mechanoreceptors within ACL remnants. Lee et al18 analyzed 36 human ACL remnants using immunohistochemical staining for NFP and observed mechanoreceptors in 33% of remnants. Adachi et al1 correlated the number of these mechanoreceptors in the tibial ACL remnant with better preoperative proprioceptive function in 29 patients. Some authors use this finding to argue for preserving the ACL tibial remnant during ACLR in an attempt to preserve proprioceptive function.19,22 In the future, biological techniques to promote reinnervation of grafts from stump remnants may have potential to improve proprioceptive function after ACLR.
Our findings support those of clinical studies showing neuromuscular control deficits persist many years after ACLR. Denti et al10 used static and dynamic tests to assess motor control function in 50 patients after bone-tendon-bone autograft and compared the results with 50 control subjects. They found significant motor control deficits persisted even 5 to 8 years post-ACLR. Ozenci et al21 compared proprioceptive function in patients undergoing ACLR with allograft or autograft and found no difference between groups. Barrett6 assessed 45 patients after ACLR and found a significant correlation between proprioceptive function and patient satisfaction, suggesting strategies to preserve such function may enhance outcome.
Limitations of this study should be noted. Consistent with previous studies, a relatively small amount of NFP+ neural tissue was detected. Sampling error may mean that mechanoreceptors were missed, particularly in patients with intact ACLs who underwent biopsy only. In an attempt to compensate for this, we harvested complete tibial stumps and took biopsy specimens at the ACL insertion, as a number of previous studies have demonstrated that ACL mechanoreceptor density is greatest at the tibial insertion.23,24 Second, while similar in size to a number of previous reports on this topic,14,15,17,24 the number of patients was relatively small. There was also only 1 hamstring autograft in the autograft group, and this study was not equipped to evaluate differences in types of autograft. There was also only 1 control patient; however, as expected, NFP+ tissue was clearly seen, confirming our processing technique was effective. Finally, for patients who underwent allograft ACLR, we lacked certain details of the original allograft with the potential to affect graft reinnervation, such as donor age and use of irradiation. Stimulation seems to be a requirement for maintenance of mechanoreceptors in native ACL tissue; however, we did not find evidence of mechanoreceptor reinnervation in biopsy specimens of patients with intact and well-functioning grafts. Lack of mechanoreceptor reinnervation in grafts is likely due to multiple factors, and may also have an effect in allograft sterilization (chemical or radiation).
Conclusion
We were unable to find evidence that significant mechanoreceptor reinnervation of ACL grafts occurs in humans. We also found no evidence of enhanced reinnervation when autografts were used. These data may explain the persistent proprioceptive deficit after ACLR. Further research into biological techniques to enhance reinnervation may offer a potential pathway to improve proprioceptive function after ACLR.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
References
- 1. Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand. 2002;73:330–334. [DOI] [PubMed] [Google Scholar]
- 2. Ardern CL, Taylor NF, Feller JA, Webster KE. Return-to-sport outcomes at 2 to 7 years after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2012;40:41–48. [DOI] [PubMed] [Google Scholar]
- 3. Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45:596–606. [DOI] [PubMed] [Google Scholar]
- 4. Bali K, Dhillon MS, Vasistha RK, Kakkar N, Chana R, Prabhakar S. Efficacy of immunohistological methods in detecting functionally viable mechanoreceptors in the remnant stumps of injured anterior cruciate ligaments and its clinical importance. Knee Surg Sports Traumatol Arthrosc. 2011;20:75–80. [DOI] [PubMed] [Google Scholar]
- 5. Barrack RL, Lund PJ, Munn BG, Wink C, Happel L. Evidence of reinnervation of free patellar tendon autograft used for anterior cruciate ligament reconstruction. Am J Sports Med. 1997;25:196–202. [DOI] [PubMed] [Google Scholar]
- 6. Barrett DS. Proprioception and function after anterior cruciate reconstruction. J Bone Joint Surg Br. 1991;73:833–837. [DOI] [PubMed] [Google Scholar]
- 7. Borsa PA, Lephart SM, Irrgang JJ, Safran MR, Fu FH. The effects of joint position and direction of joint motion on proprioceptive sensibility in anterior cruciate ligament-deficient athletes. Am J Sports Med. 1997;25:336–340. [DOI] [PubMed] [Google Scholar]
- 8. Co FH, Skinner HB, Cannon WD. Effect of reconstruction of the anterior cruciate ligament on proprioception of the knee and the heel strike transient. J Orthop Res. 1993;11:696–704. [DOI] [PubMed] [Google Scholar]
- 9. Denti M, Monteleone M, Berardi A, Panni AS. Anterior cruciate ligament mechanoreceptors. Histologic studies on lesions and reconstruction. Clin Orthop Relat Res. 1994;308:29–32. [PubMed] [Google Scholar]
- 10. Denti M, Randelli P, Vetere Lo D, Moioli M, Bagnoli I, Cawley PW. Motor control performance in the lower extremity: normals vs. anterior cruciate ligament reconstructed knees 5-8 years from the index surgery. Knee Surg Sports Traumatol Arthrosc. 2000;8:296–300. [DOI] [PubMed] [Google Scholar]
- 11. Dhillon MS, Bali K, Vasistha RK. Immunohistological evaluation of proprioceptive potential of the residual stump of injured anterior cruciate ligaments (ACL). Int Orthop. 2010;34:737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freeman MA, Wyke B. Articular contributions to limb muscle reflexes. The effects of partial neurectomy of the knee-joint on postural reflexes. Br J Surg. 1966;53:61–68. [DOI] [PubMed] [Google Scholar]
- 13. Fromm B, Kummer W. Nerve supply of anterior cruciate ligaments and of cryopreserved anterior cruciate ligament allografts: a new method for the differentiation of the nervous tissues. Knee Surg Sports Traumatol Arthrosc. 1994;2:118–122. [DOI] [PubMed] [Google Scholar]
- 14. Georgoulis AD, Pappa L, Moebius U, et al. The presence of proprioceptive mechanoreceptors in the remnants of the ruptured ACL as a possible source of re-innervation of the ACL autograft. Knee Surg Sports Traumatol Arthrosc. 2001;9:364–368. [DOI] [PubMed] [Google Scholar]
- 15. Katonis PG, Assimakopoulos AP, Agapitos MV, Exarchou EI. Mechanoreceptors in the posterior cruciate ligament. Histologic study on cadaver knees. Acta Orthop Scand. 1991;62:276–278. [DOI] [PubMed] [Google Scholar]
- 16. Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982;10:329–335. [DOI] [PubMed] [Google Scholar]
- 17. Kim SH, Chun CH, Chun KC, Jo HJ, Kim KM. Histological assessment of mechanoreceptors in Achilles allografts after anterior cruciate ligament reconstruction. Am J Sports Med. 2012;40:2061–2065. [DOI] [PubMed] [Google Scholar]
- 18. Lee BI, Min KD, Choi HS, et al. Immunohistochemical study of mechanoreceptors in the tibial remnant of the ruptured anterior cruciate ligament in human knees. Knee Surg Sports Traumatol Arthrosc. 2009;17:1095–1101. [DOI] [PubMed] [Google Scholar]
- 19. Nakase J, Toratani T, Kosaka M, Ohashi Y, Tsuchiya H. Roles of ACL remnants in knee stability. Knee Surg Sports Traumatol Arthrosc. 2013;21:2101–2106. [DOI] [PubMed] [Google Scholar]
- 20. Ochi M, Iwasa J, Uchio Y, Adachi N, Sumen Y. The regeneration of sensory neurones in the reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 1999;81:902–906. [DOI] [PubMed] [Google Scholar]
- 21. Ozenci AM, Inanmaz E, Ozcanli H, et al. Proprioceptive comparison of allograft and autograft anterior cruciate ligament reconstructions. Knee Surg Sports Traumatol Arthrosc. 2007;15:1432–1437. [DOI] [PubMed] [Google Scholar]
- 22. Papalia R, Franceschi F, Vasta S, Di Martino A, Maffulli N, Denaro V. Sparing the anterior cruciate ligament remnant: is it worth the hassle? Br Med Bull. 2012;104:91–111. [DOI] [PubMed] [Google Scholar]
- 23. Raunest J, Sager M, Bürgener E. Proprioception of the cruciate ligaments: receptor mapping in an animal model. Arch Orthop Trauma Surg. 1998;118:159–163. [DOI] [PubMed] [Google Scholar]
- 24. Schutte MJ, Dabezies EJ, Zimny ML, Happel LT. Neural anatomy of the human anterior cruciate ligament. J Bone Joint Surg Am. 1987;69:243–247. [PubMed] [Google Scholar]


