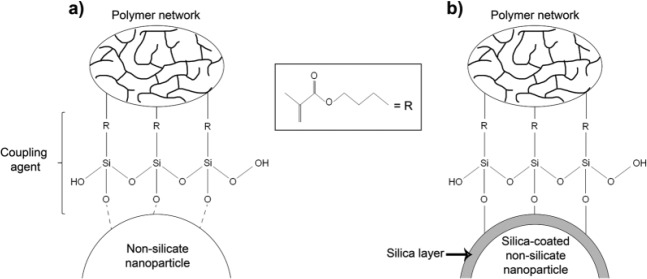
Figure 1.
Representative illustration of the interaction among self-assembled crosslinked siloxane layers formed over the nanoparticles modified or not with the silica-coating method. (a) The trialkoxysilane function of the organosilane cannot chemically bond to the nanoparticles due to the absence of silica; thus, only physical interactions with the surfaces are formed. A siloxane layer is deposited around the nanoparticles by crosslinking among the silane molecules, but the coupling with the nanoparticle is not effective or stable. (b) The presence of a silica layer around the nanoparticles, deposited by the method proposed here, enables effective and stable silanation by formation of siloxane covalent bonds with the now silica-rich surfaces. The methacrylate group on the other end of the organosilane molecules makes the fillers compatible with the polymeric matrix.