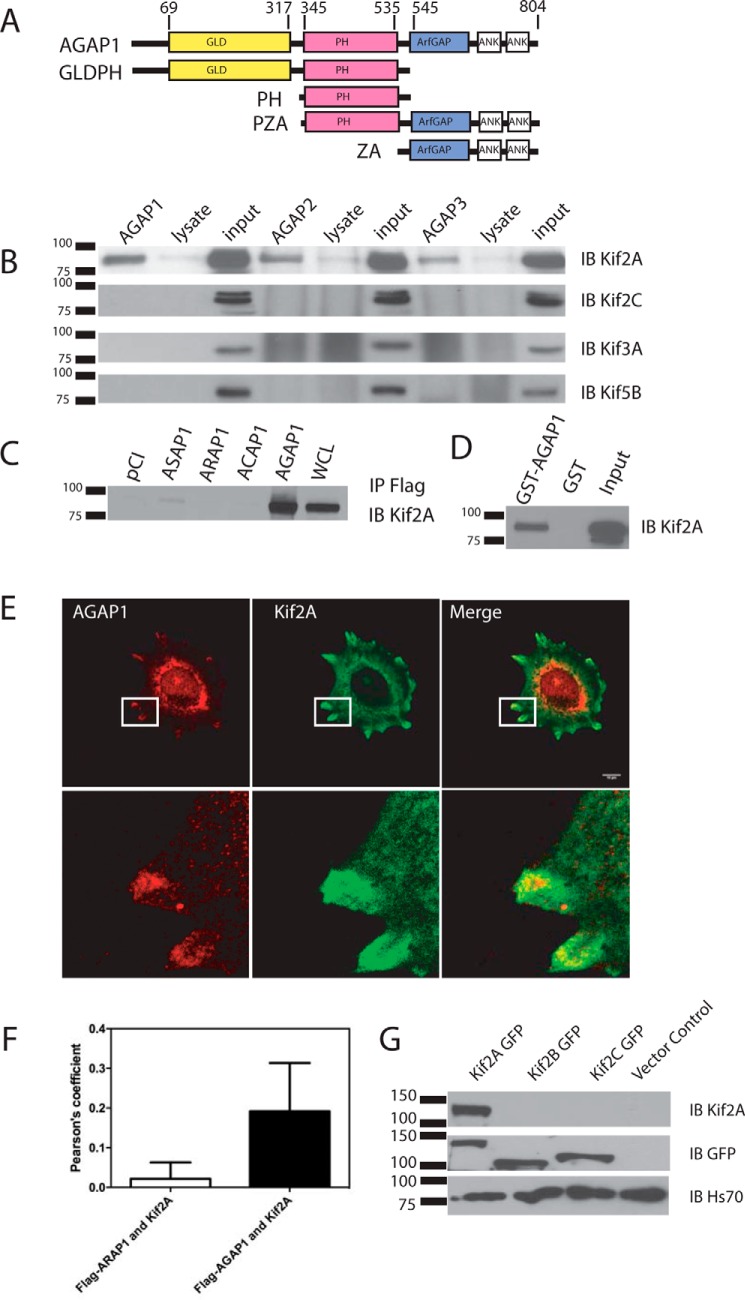
FIGURE 1.
Interaction between AGAP1 and Kif2A. A, schematic of the domain structure of AGAP1 and recombinant proteins. Ank, ankyrin repeat. B, coimmunoprecipitation of kinesins with FLAG-tagged AGAPs. HeLa cells were transfected with an empty pCI vector or pCI with cDNA for FLAG-AGAP1, FLAG-AGAP2, or myc-AGAP3. The cells were lysed after 24 h. Proteins from the lysates were immunoprecipitated with an antibody against the FLAG or myc epitope, and the precipitates were probed with an antibody against Kif2A, Kif2A, Kif3A, and Kif5B. IB, immunoblotting. C, association of GST-AGAP1 with Kif2A in a cell lysate. HeLa cell lysate was incubated with GST or GST-AGAP at 4 °C overnight. GST was precipitated with glutathione beads, and Kif2A was detected in the precipitates by immunoblotting using a polyclonal antibody against Kif2A. The total Hela cell lysate was included as a positive control. IP, immunoprecipitation. D, co-immunoprecipitation of Kif2A with four Arf GAP subtypes. HeLa cells were transfected with empty pCI vector or pCI with cDNA for FLAG-ASAP1, FLAG-ARAP1, FLAG-ACAP1, or FLAG-AGAP1. The cells were lysed after 24 h. Proteins from the lysates were immunoprecipitated with an antibody against the FLAG epitope. The precipitates were probed with an antibody against Kif2A. E, colocalization of FLAG-AGAP1 and endogenous Kif2A. Hela cells were transfected with FLAG-AGAP1 for 24 h. Cells were replated on fibronectin-coated coverslips in Opti-MEM for 6 h. The cells were fixed and stained using a monoclonal anti-FLAG antibody and a polyclonal rabbit anti-Kif2A serum. A 0.9-μm slice of the ventral surface is shown. F, quantification of colocalization of Kif2A with FLAG-AGAP1 and FLAG-ARAP1. Pearson's coefficients for Kif2A with either FLAG-AGAP1 or FLAG-ARAP1 in the periphery of the cells were determined for 30 cells (10 from each of 3 experiments). The data presented are the mean ± S.D. p < 0.0001 for FLAG-AGAP1-Kif2A. G, specificity of Kif2A antibody. Kif2A-GFP, Kif2B-GFP, and Kif2C-GFP were expressed in HeLa cells. The cell lysates were analyzed by immunoblotting using either the anti-Kif2A antibody and/or the anti-GFP antibody. Although all three proteins were expressed at similar levels, indicated by the signal with the anti-GFP antibody, signal was only observed with Kif2A when using the anti-Kif2A antibody.