FIGURE 5.
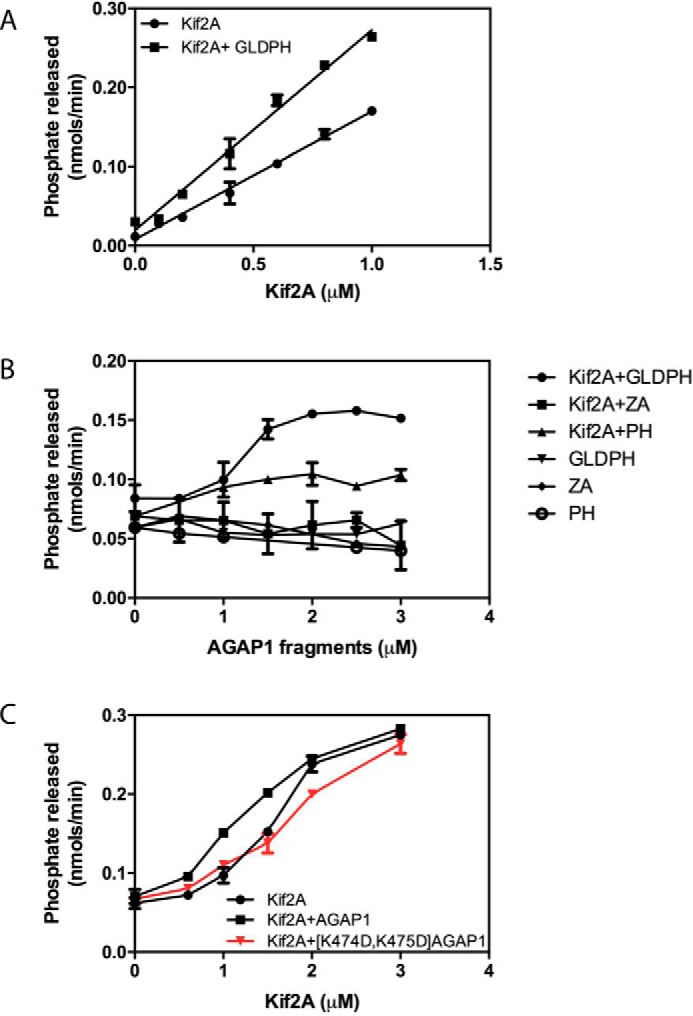
Effect of AGAP1 on Kif2A ATPase activity. A, titration of Kif2A with fixed GLDPH. Purified His10-[193–531]Kif2A was titrated into a 30-μl reaction containing 200 μm LUVs and 0.2 μg of microtubules with or without 1.0 μm of purified His10-GLDPH domain of AGAP1. The reactions were started by adding 0.3 mm ATP and stopped after 5 min. The data were analyzed by linear regression. The slope of Kif2A+GLDPH was greater than the slope of Kif2A alone, p < 0.001. B, titration of AGAP1 into a reaction with fixed Kif2A. The isolated His10-GLDPH of AGAP1 (the minimum binding domain), a recombinant protein composed of the Arf GAP and ankyrin repeats of AGAP1 fused to a 10-histidine tag (designated ZA in the figure, which does not bind to Kif2A), or the PH domain of AGAP1 was titrated into a reaction containing 200 μm LUVs, 0.2 μg of microtubules, and 0.7 μm His10-[191–531] Kif2A as indicated. Reactions were initiated by the addition of ATP and terminated after 5 min. Data were analyzed by two-way ANOVA. GLDPH increased Kif2A activity, p < 0.001; PH increased activity, p < 0.001; PH had less effect than GLDPH, p < 0.001; ZA had no significant effect. C, effect of mutation in the PH domain of AGAP1 on Kif2A ATPase activity. Purified His10-[193–531]Kif2A was titrated into a reaction mixture containing full-length AGAP1 or [K474D,K475D]AGAP1 as described for panel A. The data were analyzed by two-way ANOVA. AGAP1 plus Kif2A was greater than Kif2A alone, p < 0.001; [K474D,K475D]AGAP1 did not have a significant effect. Results shown are the mean ± S.E. from three independent experiments.
