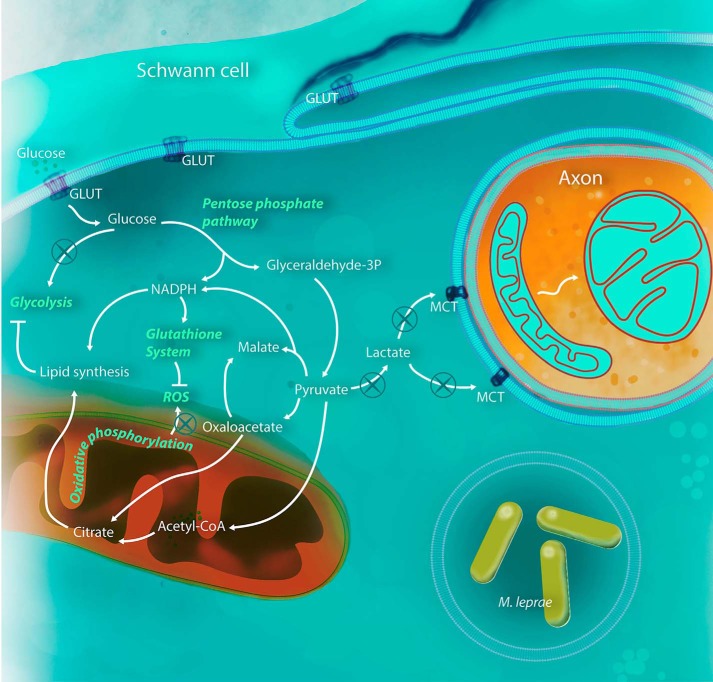
FIGURE 7.
M. leprae is able to modulate Schwann cell metabolism for its benefit. After infection, Schwann cells increase glucose uptake, feeding the PPP with carbon, in detriment to glycolysis, probably due to phosphofructokinase inhibition by the increased levels of acyl-CoA in the cytoplasm. Pyruvate generated by PPP is converted to malate and acetyl-CoA instead of lactate, compromising axonal mitochondria physiology. Pyruvate is rapidly converted to citrate, increasing lipid synthesis and virtually stopping the Schwann cell tricarboxylic acid cycle and consequently respiratory chain and mitochondria energy potential. The NADPH generated by the oxidative phase of the PPP will maintain an up-regulated lipid synthesis and glutathione system. All three pathways are crucial to the success of M. leprae infection and could be used in new host-target therapy strategies.