FIGURE 7.
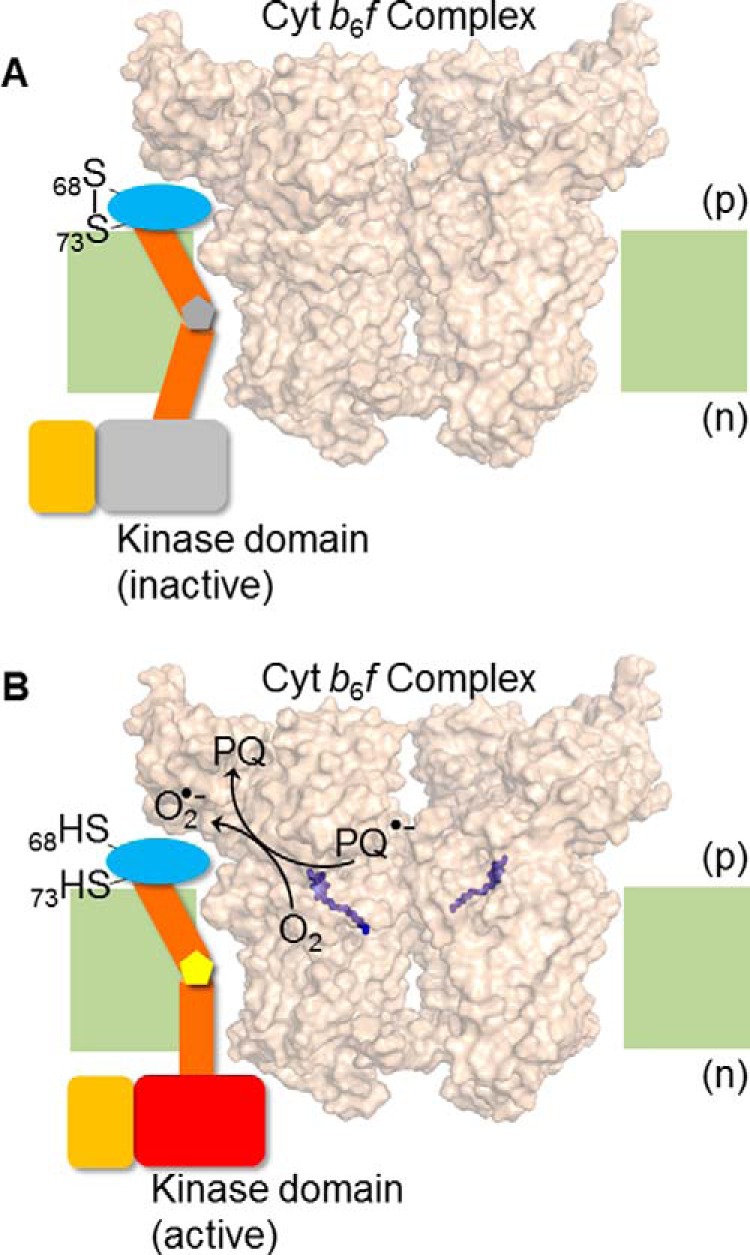
Hypothesis/model for trans-membrane activation of Stt7 kinase. Superoxide, O2˙̄, generated in the b6f complex via plastosemiquinone, reduces the disulfide bond between Cys68 and Cys73. Disulfide-sulfhydryl transition causes a conformation change in the Stt7 trans-membrane domain that is transmitted to the n side of the membrane through the proline hinge in the trans-membrane domain, thus activating the kinase on the n side. The p side domain containing Cys68 and Cys73, is shown as a blue oval, the trans-membrane domain as brown cylinders, and the proline hinge as a gray (A) and yellow (B) pentagon. The n side Stt7 kinase domain is shown in gray in the inactive state (A) and, when activated, as a red rectangle (B). The C-terminal disordered domain is shown as an orange rectangle. The cyt b6f complex (PDB code 4H13) is shown in surface representation, with the p side plastoquinol (Qp) binding site marked by the quinol analog tridecyl stigmatellin (purple sticks). Lipid bilayer, light green. (A) The Qp site of cyt b6f complex is unoccupied, Cys68 and Cys73 of Stt7 form a disulfide bond, and kinase remains inactive. (B) Upon binding and oxidation of plastoquinol at the Qp site, plastosemiquinone (PQ2˙̄) is generated which proposed to, serve as a reductant for molecular oxygen (O2), generating O2˙̄, which is oxidized to plastoquinone (PQ). As noted in the main text, the Stt7 trans-membrane domain has been proposed to occupy the niche between the peripheral F and G trans-membrane helices of subunit IV.
