FIGURE 1.
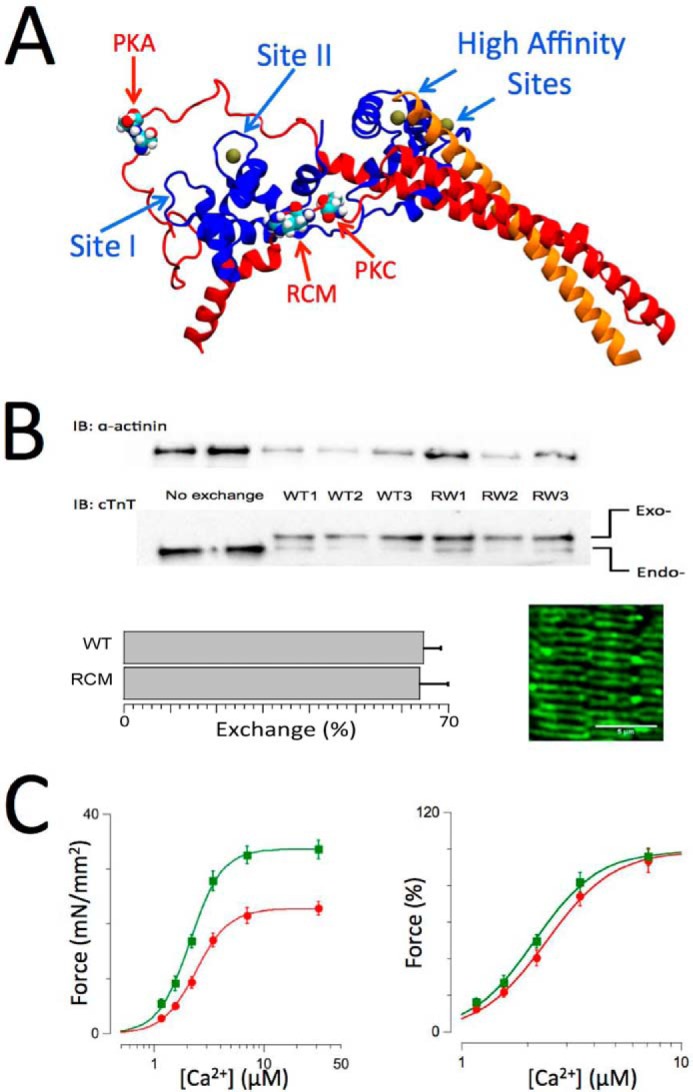
Cardiac troponin structure, exchange, and functional measurements. A, structure of troponin based on the crystal structure by Takeda et al. (46). cTnI is shown in red, cTnC is shown in blue, and cTnT is shown in orange. Ca2+ ions (solid balls) are shown bound to the high affinity sites and the single cardiac low affinity at Site II. The locations in cTnI of the PKA (Ser-23/Ser-24) and protein kinase C (Thr-143) phosphorylation motifs and the RCM mutation (R145W) are as indicated. B, endogenous troponin (Endo-) was exchanged for recombinant human troponin (Exo-) containing either WT, RCM mutation, or PKA/PKC phosphomimetic cardiac troponin I. Inclusion of an N-terminal myc tag in cTnT allowed for separation of the endogenous and exogenous cTnT on SDS-PAGE/Western blotting using a cTnT-specific antibody (bottom gels), whereas anti-α-actinin Western blotting was used as a loading control (upper gels). Exchange for recombinant troponin (in this case WT or RCM) resulted in ∼68% exchange (bar plots; mean ± S.E.; WT, n = 8; RCM, n = 12). The image shows an example of a confocal microscopy scan of a WT recombinant troponin-exchanged muscle probed with a myc-specific primary antibody followed by a mouse Alexa Fluor-488 secondary antibody; the double banded pattern indicates incorporation of the recombinant troponin into the thin filaments on either side of the Z-disk. C, force-[Ca2+] relationships (n = 12) recorded on WT recombinant troponin-exchanged isolated skinned multicellular human cardiac muscles at short (SL = 2.0 μm; red) and long (SL = 2.3 μm; green) sarcomere lengths. Force was either normalized to muscle cross-sectional area and expressed as millinewtons (mN)/mm2 (left panel) or normalized to maximum force in each individual muscle (right panel). An increase in SL induced both an increase in maximum force and an increase in myofilament Ca2+ sensitivity, indicative of myofilament LDA. Data are presented as mean ± S.E. Error bars reflect S.E. IB, immunoblotting.
