FIGURE 7.
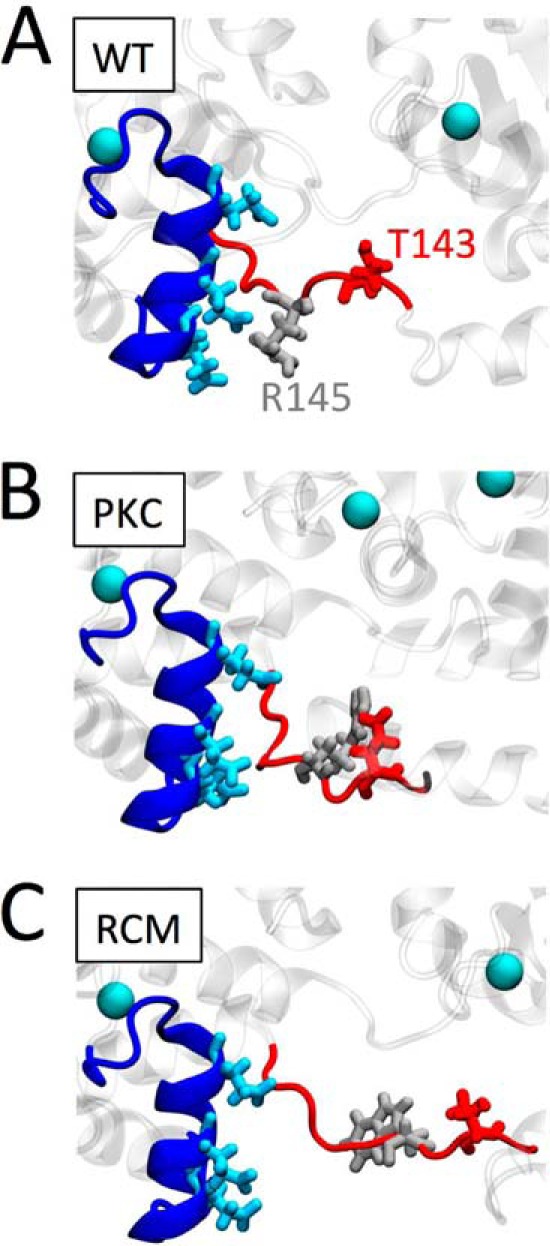
Proposed structural impact of the PKC phosphomimetic or the RCM disease mutation. Enlarged structural detail of troponin shows cTnC (dark blue), highlighting helix C Glu-56, Glu-59, and Glu-63 (light blue). cTnI is shown in red; WT Thr-143 (A and C) or the PKC phosphomimetic Glu-143 (B) is shown in dark red. WT arginine Arg-145 (A and B) or its mutation to Trp-145 is shown in light gray. Ca2+ ions are illustrated as light blue balls. Introduction of the negatively charged PKC phosphomimetic at cTnI position 143 causes this region of cTnI to sample structural states away from negatively charged Glu-56, Glu-59, and Glu-63 within the cTnC helix C domain. Replacing the positively charged arginine residue at cTnI position 145 by a neutral, relatively large tryptophan in the RCM-associated mutation further exacerbates the structural conformational changes in this region. We propose that these structural changes lead to increased myofilament Ca2+ sensitivity and uncoupling of the functional impacts mediated by cTnI PKA phosphorylation.
