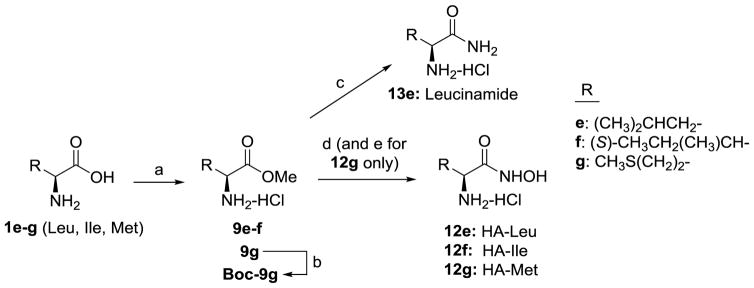
Scheme 1.
Synthesis of compounds 13e and 12e–12g. Reagents and conditions: (a) SOCl2, MeOH, 9e: 56%, 9f: 86%, 9g: 67%; (b) Boc2O, DCM, Boc-9g: 85%; (c) 7N NH3 in MeOH, 50 °C, sealed tube, 13e: 60%; (d) 50% NH2OH in water, MeOH or 1,4-dioxane, 12e: 23%, 12f: 3%, 12g: 20% (2 steps); (e) 4N HCl in 1,4-dioxane. 13e and 12e–g were purified by conversion to their HCl salts and recrystallization to >99% purity by HPLC.