Table 2.
Exchange efflux rate and uptake inhibition of [3H]-gabapentin in HEK-hLAT1 cells for hydroxamic acids, related carboxylic acid derivatives, and their parent amino acids.
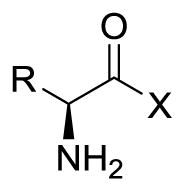
| |||||
|---|---|---|---|---|---|
| Compounda | X | R | Efflux Rateb | % Inhibitionc | IC50 (μM)d |
| 1a (Phe) | PhCH2- | 3.6 ± 0.7 | 85 ± 0.6 | - | |
| 1b (Tyr) | p-HOPhCH2- | 2.6 ± 0.4 | 68 ± 0.5 | - | |
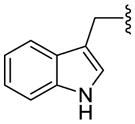
| 1d (Trp) |
|
1.6 ± 0.3 | 79 ± 0.6 | - | |
| 1e (Leu) | -OH | (CH3)2CHCH2- | 3.2 ± 0.5 | 73 ± 0.7 | 87 ± 10 |
| 1f (Ile) | (S)-CH3CH2(CH3)CH- | 2.5 ± 0.1 | - | 150 ± 40 | |
| 1g (Met) | CH3S(CH2)2- | 2.4 ± 0.1 | - | 180 ± 3 | |
| 1h (Gly) | H | 0.78 ± 0.2 | 33 ± 3 | >200 | |
| 1i (Arg) | NH2(NH)CNH(CH2)3- | 0.75 ± 0.1 | 49 ± 2 | - | |
|
| |||||
| 12a (HA-Phe) | PhCH2- | 1.3 ± 0.1 | 24 ± 0.5 | - | |
| 12b (HA-Tyr) | p-HOPhCH2- | 1.0 ± 0.1 | 39 ± 30 | - | |
| 12d (HA-Trp) |

|
1.0 ± 0.1 | 35 ± 1 | - | |
| 12e (HA-Leu) | -NHOH | (CH3)2CHCH2- | 1.5 ± 0.1 | 50. ± 1 | >200 |
| 12f (HA-Ile) | (S)-CH3CH2(CH3)CH- | 1.5 ± 0.2 | - | >200 | |
| 12g (HA-Met) | CH3S(CH2)2- | 1.1 ± 0.01 | - | >200 | |
| 12h (HA-Gly) | H | 0.70 ± 0.1 | 38 ± 1 | >200 | |
|
| |||||
| 9e (Leu ester) | -OMe | 2.1 ± 0.2 | - | >200 | |
| 13e (Leucinamide) | -NH2 | (CH3)2CHCH2- | 0.69 ± 0.01 | - | >200 |
Cell assay data was obtained at least in triplicate. Amino acids and their corresponding derivatives possess S stereochemistry at the α-carbon.
Compounds were tested at 200 μM for their ability to cause efflux (fmol/min) of [3H]-gabapentin from pre-loaded HEK-hLAT1 cells.
Compounds were tested at 200 μM for their ability to inhibit uptake of [3H]-gabapentin into HEK-hLAT1 cells. Data is presented as % inhibition relative to background signal in the absence of a test compound.
