Abstract
Background
Sex hormones play a role in gastric cancer and colorectal cancer etiology, however, epidemiological evidence is inconsistent. This study examines the influence of menstrual and reproductive factors over the risk of both tumors.
Methods
In this case-control study 128 women with gastric cancer and 1293 controls, as well as 562 female and colorectal cancer cases and 1605 controls were recruited in 9 and 11 Spanish provinces, respectively. Population controls were frequency matched to cases by age and province. Demographic and reproductive data were directly surveyed by trained staff. The association with gastric, colon and rectal cancer was assessed using logistic and multinomial mixed regression models.
Results
Our results show an inverse association of age at first birth with gastric cancer risk (five-year trend: OR = 0.69; p-value = 0.006). Ever users of hormonal contraception presented a decreased risk of gastric (OR = 0.42; 95%CI = 0.26–0.69), colon (OR = 0.64; 95%CI = 0.48–0.86) and rectal cancer (OR = 0.61; 95%CI = 0.43–0.88). Postmenopausal women who used hormone replacement therapy showed a decreased risk of colon and rectal tumors. A significant interaction of educational level with parity and months of first child lactation was also observed.
Conclusion
These findings suggest a protective role of exogenous hormones in gastric and colorectal cancer risk. The role of endogenous hormones remains unclear.
Introduction
Colorectal cancer (CRC) in Spain, with an estimated 16071 new cases in 2014 [1], represents the second most common tumor in women and the second leading cause of death, accounting for 15.3% of all female cancer-related deaths in 2013 [2]. In Spain, as in other developed countries [3], there has been an increase in incidence due to this type of cancer, slightly attenuated around 1995 [4]. However, mortality rates have reached a plateau since the beginning of this century [5].
Gastric cancer (GC) in Spanish women occupies the tenth position in incidence, with an adjusted rate of 7.5 cases per 100,000 estimated for 2012 [6]. In terms of mortality, the rate estimated for this same year was 4.8 per 100,000, accounting for 5.3% of all female cancer-related deaths in 2013 [2]. The small difference between the incidence and mortality rates is due to the low survival recorded for this tumor, which is estimated in Spain to be 26.0% at 5 years [1].
Hormonal factors may play a role in the etiology of both tumors: incidence is approximately twofold higher among males than among females [6], even though differential exposure to established risk factors cannot totally explain these differences. On the other hand, it has been suggested that estrogens may offer protection against the development of both tumors, and this protective effect seems to be modulated through estrogen receptors (ER) identified in non-cancerous and cancerous gastric [7] and colonic tissue [8, 9]. Recent studies indicate that the protection conferred to CRC risk could be limited to certain molecular tumor subtypes [8] and to exogenous estrogens exposure [9, 10]. On the contrary, while some studies have observed lower GC risk associated with the exposure to estrogens of both ovarian and exogenous origin [11], other authors have detected this association with endogenous estrogen exposure only [12].
The role of menstrual and reproductive factors in the etiology of GC, and mainly CRC, has drawn interest in the literature, but findings are not consistent, especially in the case of GC where most studies have been limited by small number of cases. This study sought to investigate the influence of menstrual and reproductive factors on female GC and CRC risk in a large population-based case control study in Spain. We also assessed if these associations differ by specific CRC subsite. Finally, taking into account that reproductive patterns and the use of hormonal compounds are strongly influenced by womens’ educational level [13] and that highly educated women tend to have healthier life-styles [14, 15], we evaluated the influence of reproductive factors separately in women with high and low educational background.
Materials and Methods
Study population
Multicase-control Spain study (MCC-Spain, www.mccspain.org) [16], with population controls and incident cases was carried out between September 2008 and December 2013 to investigate the influence of environmental factors and their interaction with genetic factors in highly prevalent tumors or with peculiar epidemiological characteristics in Spain. Cases of breast, prostate, gastric, colorectal tumors and chronic lymphocytic leukemias were recruited from 23 hospitals in 12 Spanish provinces. Inclusion criteria required that participants should have resided for at least 6 months in the study areas, had to be aged 20–85 and had to be mentally qualified to answer the questionnaire. A group of controls, common for the five types of tumors, was randomly selected from the administrative records of a number of primary care health centers within the catchment areas of the hospitals where cases were recruited. We made an initial estimate of the age-sex distribution that cases–all combined- would have in each region, according to the tumors they recruited and to the cancer incidence rates from Spanish cancer registries. We applied these estimates to predefine the age-sex distribution of our population-based controls, which were selected randomly from the general practitioner lists of each hospital catchment area. When the recruitment of cases ended, we compared again the age- sex- distribution of cases and controls and recruited new participants if needed in an attempt to ensure that each case had at least one control of the same 5-year age interval and sex in each region. Controls were initially contacted via telephone and those who agreed to participate in the study were scheduled for a personal interview. Cases of GC were recruited in Madrid, Barcelona, León, Navarra, Cantabria, Asturias, Huelva, Valencia and Granada. CRC cases were also recruited in Guipúzcoa and Murcia. Our research personnel actively searched for new cases, through regular visits to the collaborating hospital departments (gastroenterology, oncology, general surgery, radiotherapy and pathology) and reviewed the hospital admission registries weekly.
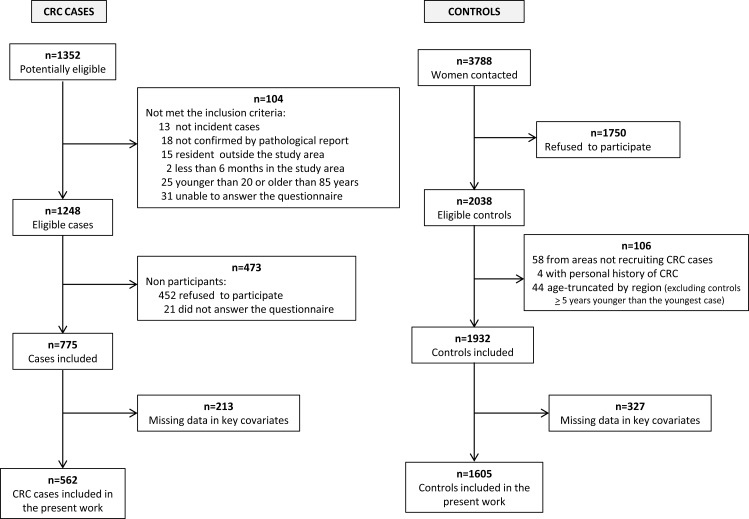
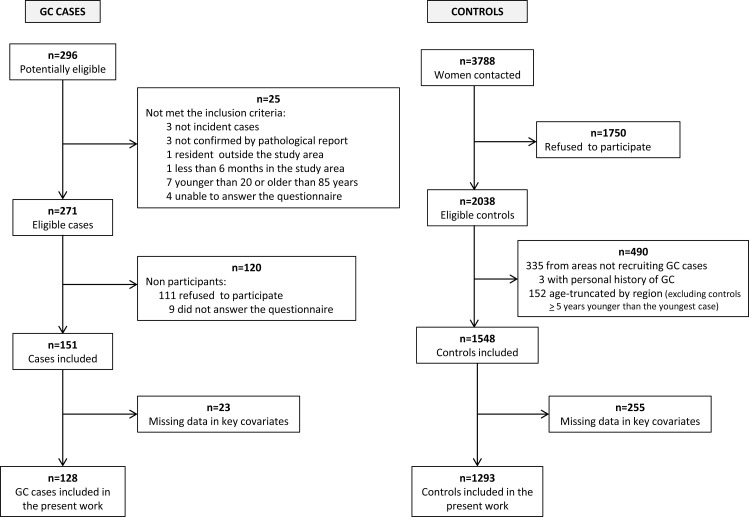
For the present study we recruited a total of 151 histologically-confirmed female GC cases (codes C16:Malignant neoplasm of stomach and D00.2:Carcinoma in situ of stomach, according to the 10th revision of the International Statistical Classification of Diseases), and 775 female CRC cases (codes C18:Malignant neoplasm of colon, C19:Malignant neoplasm of rectosigmoid junction, C20:Malignant neoplasm of rectum, D01.0:Carcinoma in situ of colon, D01.1:Carcinoma in situ of rectosigmoid junction and D01.2:Carcinoma in situ of rectum) with no prior history of the disease and diagnosed within the recruitment period. Those controls with a history of GC or CRC were excluded, as well as those who resided in provinces that had not recruited these tumors and those that were more than five years younger than the youngest case included in each region. A total of 1548 and 1932 female controls were included for the GC and CRC analyses, respectively. Flow charts displaying the selection process of CRC cases and controls and GC cases and control are shown in Fig 1 and Fig 2 respectively.
Fig 1. Flow chart displaying the selection process of colorectal cancer cases and controls.
MCC-Spain study 2008–2013.
Fig 2. Flow chart displaying the selection process of gastric cancer cases and controls.
MCC-Spain study 2008–2013.
Ethical approval
The protocol of MCC-Spain was approved by Ethics Committees of the participating institutions: Comité Ético de Investigación Clínica (CEIC) del Instituto Municipal de Asistencia Sanitaria de Barcelona; CEIC del Hospital Universitario de Bellvitge; CEIC de Navarra; CEIC del Hospital Universitario La Paz; CEIC del Hospital Universitario Ramón y Cajal; CEIC de Cantabria; CEIC de la Dirección General de Salud Pública y Centro Superior de Investigación en Salud Pública; CEIC del Hospital General Universitario Jose Mª Morales Meseguer; Comité de Ética de la Investigación de la Provincia de Huelva; CEIC de León; Comité Ético de Investigación del Principado de Asturias; Comité de Ética de la Investigación Biomédica Provincial de Granada; Comité de Ética en Investigación Humana de la Universidad de Granada; Comité Ético de Investigación de la Comunidad Autónoma del País Vasco. All participants were informed about the study objectives and signed an informed consent. Confidentiality of data is secured removing personal identifiers in the datasets. The database was registered in the Spanish Agency for Data Protection, number 2102672171.
Data collection
Exposure information was collected by trained interviewers through face-to-face interviews using a structured electronic questionnaire, including detailed information on demographic factors, occupation, personal and family history, lifestyle and diet [17]. Menstrual factors gathered by the questionnaire included age at menarche, regularity of the menstrual cycle, menopausal status, age and cause of menopause, use of hormonal contraceptives and hormone replacement therapy. Regarding reproductive history, the questionnaire collected information on fertility problems and their treatment, number of miscarriages, number of children, newborns' sex, year of birth, gestational age and duration of maternal lactation.
Statistical analyses
Descriptive analyses of participants’ characteristics were done for GC, colon cancer (CC) and rectal cancer (RC) cases and controls. We used absolute figures and percentages to describe categorical variables, and means and standard deviations to describe continuous variables. Significant differences between cases and controls were tested using Pearson chi-square and Student's t-test for categorical and continuous variables respectively.
The association of menstrual and reproductive variables with GC, CC and RC was assessed using logistic mixed regression models, including the province as a random effect term to account for unexplained heterogeneity associated with province, such as distribution of unmeasured potential risk factors or differences between interviewers. Models were adjusted for age, educational level, body mass index (BMI) one year prior to the interview, tobacco consumption, family history of the studied cancer, hormone replacement therapy use and hormonal contraception use. We also conducted an additional analysis in CRC cases and controls stratifying by educational level, not done for GC due to the low number of cases. Finally, a sensitivity analysis was done additionally adjusting for calorie intake, red meat, processed meat, fruits, vegetables and alcohol consumption in those participants who fulfilled the food frequency questionnaire (Supporting Information).
CRC patients were classified according to tumor subsite: CC and RC (two cases were of unspecified subsite). Since our response variable has three categories (0 = controls, 1 = CC cases and 2 = RC cases), we fitted multinomial logistic regression models to evaluate the association of menstrual and reproductive factors with the above-mentioned CRC subsites. These models were adjusted by the same set of variables described above, including the province as a random effect term. Heterogeneity of effects was tested using a Wald test to compare the coefficients obtained for the different CRC subsites. Data were analyzed using STATA/MP 13.1 software.
Results
Response rates were 53.8% for healthy female controls, 62.1% for female CRC cases and 55.7% for female GC cases. Results presented in this manuscript are based on women with no missing values in any of the selected confounders (73% of CRC cases, 85% of GC cases and 83% of controls). We included 562 CRC cases (364 colon cases and 196 rectal cases) and 1605 controls, as well as 128 GC cases and 1293 controls. Table 1 shows the main characteristics of these women. In general, gastric, colon and rectal cases were older and had higher BMI than controls. They also smoked less, had lower educational level, had a delayed menarche, had more children, had their first child at a younger age, had longer breastfeeding periods and reported to use less hormone replacement therapy and hormonal contraception than controls.
Table 1. Socio-demographic, menstrual and reproductive characteristics in gastric and colorectal cancer cases and controls.
| GASTRIC CANCER | COLORECTAL CANCER | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Cases | Controls | Colon cases | Rectal cases | ||||||||||
| (N = 1293) | (N = 128) | (N = 1605) | (N = 364) | (N = 196) | ||||||||||
| N | (%) | N | (%) | p-val | N | (%) | N | (%) | p-val | N | (%) | p-val | ||
| Age, mean(SD) | 59.5 | (12.6) | 65.0 | (14.2) | <0.001 | 58.9 | (12.5) | 65.5 | (11.4) | <0.001 | 64.3 | (11.8) | <0.001 | |
| Educational level | <0.001 | <0.001 | <0.001 | |||||||||||
| Less than primary school | 197 | (15%) | 52 | (41%) | 259 | (16%) | 108 | (30%) | 65 | (33%) | ||||
| Primary school completed | 422 | (33%) | 47 | (37%) | 490 | (31%) | 140 | (38%) | 74 | (38%) | ||||
| Secondary school | 392 | (30%) | 24 | (19%) | 495 | (31%) | 73 | (20%) | 35 | (18%) | ||||
| University graduate | 282 | (22%) | 5 | (4%) | 361 | (22%) | 43 | (12%) | 22 | (11%) | ||||
| BMI | 0.003 | <0.001 | 0.045 | |||||||||||
| <20 Kg/m2 | 91 | (7%) | 5 | (4%) | 111 | (7%) | 15 | (4%) | 13 | (7%) | ||||
| 20–24 Kg/m2 | 542 | (42%) | 36 | (28%) | 681 | (42%) | 116 | (32%) | 64 | (33%) | ||||
| 25–29 Kg/m2 | 409 | (32%) | 50 | (39%) | 506 | (32%) | 139 | (38%) | 78 | (40%) | ||||
| >29 Kg/m2 | 251 | (19%) | 37 | (29%) | 307 | (19%) | 94 | (26%) | 41 | (21%) | ||||
| Smoking | 0.001 | <0.001 | 0.004 | |||||||||||
| Never smoker | 778 | (60%) | 97 | (76%) | 934 | (58%) | 257 | (71%) | 135 | (69%) | ||||
| Former smoker > 1.5 years | 257 | (20%) | 10 | (8%) | 332 | (21%) | 56 | (15%) | 38 | (19%) | ||||
| Smoker or former smoker <1.5 years | 258 | (20%) | 21 | (16%) | 339 | (21%) | 51 | (14%) | 23 | (12%) | ||||
| Family history colorectal/gastric cancer | <0.001 | <0.001 | 0.005 | |||||||||||
| None | 1160 | (90%) | 90 | (70%) | 1397 | (87%) | 283 | (78%) | 160 | (82%) | ||||
| Second degree only | 77 | (6%) | 9 | (7%) | 67 | (4%) | 15 | (4%) | 7 | (4%) | ||||
| 1 first degree | 53 | (4%) | 20 | (16%) | 129 | (8%) | 55 | (15%) | 23 | (12%) | ||||
| >1 first degree | 3 | (0%) | 9 | (7%) | 12 | (1%) | 11 | (3%) | 6 | (3%) | ||||
| Age at menarche, mean(SD) | 12.8 | (1.7) | 13.2 | (2.0) | 0.018 | 12.8 | (1.6) | 13.0 | (1.7) | 0.024 | 13.2 | (1.6) | 0.006 | |
| Number of children | 0.006 | 0.001 | 0.047 | |||||||||||
| None | 235 | (18%) | 22 | (17%) | 278 | (17%) | 43 | (12%) | 27 | (14%) | ||||
| 1–2 | 716 | (55%) | 55 | (43%) | 902 | (56%) | 190 | (52%) | 99 | (51%) | ||||
| 3–4 | 279 | (22%) | 39 | (30%) | 350 | (22%) | 103 | (28%) | 58 | (30%) | ||||
| >4 | 61 | (5%) | 12 | (9%) | 72 | (4%) | 28 | (8%) | 12 | (6%) | ||||
| Age at first birth, mean(SD) | 26.7 | (4.8) | 24.8 | (4.7) | <0.001 | 26.8 | (4.8) | 26.2 | (4.5) | 0.040 | 26 | (5.2) | 0.051 | |
| Lactation first child (months), mean(SD) | 4.5 | (5.7) | 5.5 | (5.8) | 0.113 | 4.4 | (5.6) | 5.4 | (6.1) | 0.008 | 5.6 | (6.1) | 0.020 | |
| History of miscarriages | 0.768 | 0.013 | 0.597 | |||||||||||
| None | 985 | (76%) | 99 | (77%) | 1231 | (77%) | 301 | (83%) | 147 | (75%) | ||||
| One or more | 308 | (24%) | 29 | (23%) | 374 | (23%) | 63 | (17%) | 49 | (25%) | ||||
| Menopausal status | 0.001 | <0.001 | <0.001 | |||||||||||
| Premenopausal | 340 | (26%) | 17 | (13%) | 456 | (28%) | 39 | (11%) | 29 | (15%) | ||||
| Posmenopausal | 953 | (74%) | 111 | (87%) | 1149 | (72%) | 325 | (89%) | 167 | (85%) | ||||
| Hormonal contraception use | <0.001 | <0.001 | <0.001 | |||||||||||
| Never | 668 | (52%) | 101 | (79%) | 809 | (50%) | 254 | (70%) | 135 | (69%) | ||||
| Ever | 625 | (48%) | 27 | (21%) | 796 | (50%) | 110 | (30%) | 61 | (31%) | ||||
| Postmenopausal women | ||||||||||||||
| Hormone therapy use | 0.121 | <0.001 | 0.016 | |||||||||||
| Never | 858 | (90%) | 105 | (95%) | 1025 | (89%) | 311 | (96%) | 159 | (95%) | ||||
| Ever | 95 | (10%) | 6 | (5%) | 124 | (11%) | 14 | (4%) | 8 | (5%) | ||||
| Age at menopause, mean(SD) | 48.4 | (5.3) | 47.9 | (5.6) | 0.397 | 48.6 | (5.3) | 48.7 | (5.2) | 0.667 | 48.7 | (5.0) | 0.663 | |
| Fertility time (years), mean(SD) | 35.5 | (5.4) | 34.7 | (5.7) | 0.183 | 35.7 | (5.4) | 35.7 | (5.2) | 0.949 | 35.5 | (4.9) | 0.638 | |
Table 2 shows the association between GC, CC and RC risk and menstrual and reproductive factors. Age at first birth displayed an inverse association with GC, decreasing the risk by 31% for every five years increase in age at first birth (P = 0.006) (>29 years vs. <25: OR = 0.52; 95%CI = 0.28–0.97). This result remained significant even after adjustment for number of children (five-year trend: OR = 0.71; P = 0.014) or additionally adjusting for calorie intake, red meat, processed meat, fruits, vegetables and alcohol consumption (five-year trend: OR = 0.71; P = 0.046) (S1 Table). Women who ever used hormonal contraception showed a decreased gastric (OR = 0.42; 95%CI = 0.26–0.69), colon (OR = 0.64; 95%CI = 0.48–0.86), and rectal (OR = 0.61; 95%CI = 0.43–0.88) cancer risk, results that are confirmed after adjustment for calorie intake, red meat, processed meat, fruits, vegetables and alcohol consumption. The protection conferred by hormonal contraception use was stronger for those who used it more than 5 years for all cancer types (OR for GC = 0.26; 95%CI = 0.10–0.67; OR for CC = 0.59; 95%CI = 0.38–0.92; OR for RC = 0.53; 95%CI = 0.30–0.95). Finally, postmenopausal women who ever used hormone replacement therapy showed a decreased CC risk (OR = 0.44; 95%CI = 0.24–0.78) and almost significant decreased RC risk (OR = 0.51; 95%CI = 0.24–1.07). No significant differences were detected between colon and rectal tumors in any of the variables analyzed. Also, no significant associations were observed between GC, CC and RC risk and other menstrual and reproductive variables (history of miscarriages, age at menopause, fertility time, fertility time without children, time since last child, time since menopause, and each woman's cumulative lifetime lactation) (data not shown).
Table 2. Association between menstrual and reproductive characteristics and gastric and colorectal cancer risk.
| GASTRIC CANCER | COLORECTAL CANCER | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COLON CANCER | RECTAL CANCER | |||||||||||||||||||||
| Variablea | controls | cases | ORb | 95% CI | p-val | controls | cases | ORb | 95% CI | p-val | cases | ORb | 95% CI | p-val | P-int.c | |||||||
| Premenopausal status | 340 | 17 | 0.94 | 0.45 | - | 2.00 | 0.882 | 456 | 39 | 0.65 | 0.41 | - | 1.03 | 0.068 | 29 | 0.82 | 0.47 | - | 1.45 | 0.500 | 0.494 | |
| Nulliparity | 235 | 22 | 1.12 | 0.65 | - | 1.92 | 0.682 | 278 | 43 | 0.78 | 0.53 | - | 1.13 | 0.184 | 27 | 0.95 | 0.60 | - | 1.50 | 0.824 | 0.464 | |
| Parous women | ||||||||||||||||||||||
| Age at first birth (years) | ||||||||||||||||||||||
| <25 | 369 | 58 | 1.00 | 437 | 126 | 1.00 | 80 | 1.00 | ||||||||||||||
| 25–29 | 409 | 31 | 0.52 | 0.32 | - | 0.86 | 0.010 | 533 | 129 | 0.84 | 0.63 | - | 1.13 | 0.242 | 58 | 0.62 | 0.43 | - | 0.90 | 0.013 | ||
| >29 | 273 | 16 | 0.52 | 0.28 | - | 0.97 | 0.040 | 346 | 64 | 0.79 | 0.55 | - | 1.14 | 0.207 | 30 | 0.63 | 0.39 | - | 1.00 | 0.052 | ||
| Five-year trend* | 0.69 | 0.53 | - | 0.90 | 0.006 | 0.95 | 0.82 | 1.10 | 0.473 | 0.95 | 0.78 | 1.14 | 0.556 | 0.978 | ||||||||
| No. of children | ||||||||||||||||||||||
| 1–2 | 716 | 55 | 1.00 | 902 | 190 | 1.00 | 99 | 1.00 | ||||||||||||||
| 3–4 | 279 | 39 | 1.27 | 0.78 | - | 2.04 | 0.337 | 350 | 103 | 0.96 | 0.72 | - | 1.29 | 0.810 | 58 | 1.11 | 0.77 | - | 1.62 | 0.574 | ||
| > 4 | 61 | 12 | 1.77 | 0.82 | - | 3.82 | 0.144 | 72 | 28 | 1.09 | 0.66 | - | 1.80 | 0.743 | 12 | 0.99 | 0.50 | - | 1.96 | 0.977 | ||
| Trend per child* | 1.13 | 0.96 | - | 1.33 | 0.145 | 1.04 | 0.94 | 1.15 | 0.454 | 0.97 | 0.85 | 1.11 | 0.703 | 0.394 | ||||||||
| Lactation first child (months) | ||||||||||||||||||||||
| None | 185 | 23 | 1.40 | 0.80 | - | 2.48 | 0.241 | 241 | 58 | 1.04 | 0.72 | - | 1.48 | 0.841 | 34 | 1.29 | 0.82 | - | 2.02 | 0.274 | ||
| 1–6 | 542 | 43 | 1.00 | 682 | 141 | 1.00 | 67 | 1.00 | ||||||||||||||
| >6 | 177 | 28 | 1.53 | 0.89 | - | 2.65 | 0.126 | 217 | 80 | 1.26 | 0.89 | - | 1.77 | 0.192 | 44 | 1.43 | 0.93 | - | 2.21 | 0.105 | ||
| Six-month trend* | 1.10 | 0.89 | - | 1.35 | 0.385 | 1.10 | 0.95 | 1.26 | 0.199 | 1.09 | 0.92 | 1.30 | 0.294 | 0.987 | ||||||||
| No. of miscarriages | ||||||||||||||||||||||
| None | 985 | 99 | 1.00 | 1231 | 301 | 1.00 | 147 | 1.00 | ||||||||||||||
| One or more | 308 | 29 | 1.21 | 0.76 | - | 1.93 | 0.416 | 374 | 63 | 0.75 | 0.55 | - | 1.03 | 0.073 | 49 | 1.23 | 0.86 | - | 1.76 | 0.246 | 0.089 | |
| Age at menarche (years) | ||||||||||||||||||||||
| <12 | 254 | 24 | 1.25 | 0.72 | - | 2.16 | 0.430 | 320 | 70 | 1.15 | 0.83 | - | 1.61 | 0.400 | 33 | 0.94 | 0.61 | - | 1.46 | 0.791 | ||
| 12–13 | 599 | 46 | 1.00 | 749 | 144 | 1.00 | 82 | 1.00 | ||||||||||||||
| >13 | 433 | 56 | 1.26 | 0.81 | - | 1.96 | 0.298 | 523 | 146 | 1.17 | 0.89 | - | 1.53 | 0.267 | 80 | 1.15 | 0.81 | - | 1.61 | 0.438 | ||
| Trend per year* | 1.05 | 0.94 | - | 1.17 | 0.397 | 1.01 | 0.94 | 1.09 | 0.713 | 1.07 | 0.97 | - | 1.17 | 0.155 | 0.319 | |||||||
| Hormonal contraception use | ||||||||||||||||||||||
| Never | 668 | 101 | 1.00 | 809 | 254 | 1.00 | 135 | 1.00 | ||||||||||||||
| Ever | 625 | 27 | 0.42 | 0.26 | - | 0.69 | 0.001 | 796 | 110 | 0.64 | 0.48 | - | 0.86 | 0.003 | 61 | 0.61 | 0.43 | - | 0.88 | 0.009 | 0.820 | |
| < = 5 years | 255 | 16 | 0.62 | 0.34 | - | 1.13 | 0.121 | 315 | 47 | 0.69 | 0.48 | - | 1.00 | 0.052 | 27 | 0.68 | 0.42 | - | 1.09 | 0.108 | ||
| >5 years | 191 | 5 | 0.26 | 0.10 | - | 0.67 | 0.006 | 249 | 30 | 0.59 | 0.38 | - | 0.92 | 0.020 | 16 | 0.53 | 0.30 | - | 0.95 | 0.034 | ||
| Not known | 179 | 6 | 0.31 | 0.13 | - | 0.75 | 0.009 | 232 | 33 | 0.63 | 0.41 | - | 0.97 | 0.036 | 18 | 0.60 | 0.35 | - | 1.04 | 0.069 | ||
| Postmenopausal women | ||||||||||||||||||||||
| Age at menopause (years) | ||||||||||||||||||||||
| < = 45 | 208 | 17 | 0.68 | 0.35 | - | 1.33 | 0.260 | 245 | 57 | 0.81 | 0.55 | - | 1.19 | 0.283 | 40 | 1.15 | 0.72 | - | 1.84 | 0.567 | ||
| 46–49 | 226 | 31 | 1.07 | 0.60 | - | 1.92 | 0.821 | 259 | 78 | 1.02 | 0.71 | - | 1.47 | 0.902 | 32 | 0.87 | 0.53 | - | 1.44 | 0.595 | ||
| 50–52 | 238 | 31 | 1.00 | 294 | 90 | 1.00 | 45 | 1.00 | ||||||||||||||
| >52 | 167 | 15 | 0.70 | 0.36 | - | 1.39 | 0.312 | 212 | 64 | 0.92 | 0.63 | - | 1.34 | 0.661 | 29 | 0.83 | 0.50 | - | 1.38 | 0.464 | ||
| Five-year trend* | 0.92 | 0.75 | - | 1.14 | 0.451 | 1.00 | 0.87 | - | 1.13 | 0.947 | 0.99 | 0.83 | - | 1.18 | 0.910 | 0.955 | ||||||
| Fertility time (years) | ||||||||||||||||||||||
| <33 | 202 | 28 | 1.00 | 236 | 73 | 1.00 | 36 | 1.00 | ||||||||||||||
| 33–36 | 233 | 23 | 0.75 | 0.40 | - | 1.41 | 0.371 | 269 | 82 | 0.98 | 0.67 | - | 1.43 | 0.916 | 46 | 1.09 | 0.68 | - | 1.77 | 0.715 | ||
| 37–39 | 213 | 22 | 0.75 | 0.40 | - | 1.43 | 0.386 | 263 | 69 | 0.83 | 0.56 | - | 1.22 | 0.340 | 33 | 0.79 | 0.47 | - | 1.32 | 0.366 | ||
| >39 | 185 | 19 | 0.82 | 0.43 | - | 1.59 | 0.565 | 233 | 64 | 0.82 | 0.55 | - | 1.23 | 0.340 | 30 | 0.77 | 0.45 | - | 1.31 | 0.333 | ||
| Five-year trend* | 0.91 | 0.75 | - | 1.12 | 0.379 | 0.99 | 0.87 | - | 1.12 | 0.871 | 0.94 | 0.80 | - | 1.11 | 0.463 | 0.588 | ||||||
| Hormone therapy use | ||||||||||||||||||||||
| Never | 858 | 105 | 1.00 | 1025 | 311 | 1.00 | 159 | 1.00 | ||||||||||||||
| Ever | 95 | 6 | 0.63 | 0.26 | - | 1.53 | 0.306 | 124 | 14 | 0.44 | 0.24 | - | 0.78 | 0.005 | 8 | 0.51 | 0.24 | - | 1.07 | 0.075 | 0.743 | |
a Totals do not add up because of missing values.
b Odds ratios (ORs) and 95% confidence intervals (95% CI) adjusted for age, educational level, BMI 1-year prior to the interview, family history of gastric/colorectal cancer, tobacco, hormone therapy use and hormonal contraception use (the latter two variables were excluded as confounders when analyzing their association with gastric and colorectal cancer risk). Province was included as a random effect term.
c P-int.: P value of the interaction term between tumor subsite and the corresponding variable.
* In italics: ORs, 95% CI and P values obtained with the corresponding variable as a continuous term.
Table 3 presents the results of these associations with CRC risk stratified by educational background. Number of children and months of lactation displayed different effects in women with low and high educational level, with the interaction term proving statistically significant (P = 0.001 and 0.040 respectively): while parity showed a positive trend among women with low educational level (primary school or less), in women with higher level of education (secondary school or university) the OR decreased by 23% for every child (P = 0.012). With respect to months of lactation, whereas those women with lower educational background who breastfed their first child for longer periods registered an increased CRC risk (>6 months vs. 1–6 months: OR = 1.55; 95%CI = 1.10–2.18; P trend = 0.031), those with higher education, showed the opposite effect, although this association was not statistically significant, probably due to the small number of cases who breastfed more than six months. The protection conferred by hormonal contraception and hormone replacement therapy use was in evidence in both groups, although it was stronger for women with low educational level. While duration of hormonal contraception use was not associated with CRC risk in women with low educational level, in those with higher educational background the risk decreased by 28% for every 5-year increase in hormonal contraception use (P = 0.032) (data not shown). Finally we also detected a U-shape association with age at menarche among women with higher educational level (<12 years vs. 12–13: OR = 1.51; 95%CI = 0.98–2.33; >13 years vs. 12–13: OR = 1.57; 95%CI = 1.06–2.34). These analyses were repeated additionally adjusting for the above mentioned dietary variables, but this led to no change in the results (S2 Table).
Table 3. Association between menstrual and reproductive characteristics and colorectal cancer risk by educational level.
| COLORECTAL CANCER | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary school or less | Secondary school or University | |||||||||||||||
| Variablea | controls | cases | ORb | 95% CI | p-val | controls | cases | ORb | 95% CI | p-val | P-int.c | |||||
| Premenopausal status | 99 | 27 | 0.86 | 0.51 | - | 1.46 | 0.575 | 357 | 42 | 0.65 | 0.42 | - | 1.01 | 0.054 | 0.360 | |
| Nulliparity | 70 | 29 | 0.71 | 0.44 | - | 1.15 | 0.167 | 208 | 42 | 0.95 | 0.64 | - | 1.41 | 0.798 | 0.366 | |
| Parous women | ||||||||||||||||
| Age at first birth (years) | ||||||||||||||||
| <25 | 277 | 164 | 1.00 | 160 | 42 | 1.00 | ||||||||||
| 25–29 | 276 | 136 | 0.77 | 0.57 | - | 1.04 | 0.091 | 257 | 52 | 0.72 | 0.45 | - | 1.15 | 0.168 | ||
| >29 | 117 | 57 | 0.75 | 0.51 | - | 1.11 | 0.152 | 229 | 37 | 0.68 | 0.41 | - | 1.13 | 0.138 | ||
| Five-year trend* | 0.97 | 0.83 | - | 1.13 | 0.671 | 0.91 | 0.74 | - | 1.11 | 0.353 | 0.629 | |||||
| No. of children | ||||||||||||||||
| 1–2 | 420 | 184 | 1.00 | 482 | 106 | 1.00 | ||||||||||
| 3–4 | 212 | 139 | 1.27 | 0.94 | - | 1.70 | 0.116 | 138 | 22 | 0.54 | 0.32 | - | 0.90 | 0.019 | ||
| > 4 | 46 | 35 | 1.33 | 0.81 | - | 2.20 | 0.262 | 26 | 5 | 0.49 | 0.18 | - | 1.38 | 0.180 | ||
| Trend per child* | 1.10 | 0.99 | - | 1.21 | 0.072 | 0.77 | 0.63 | - | 0.94 | 0.012 | 0.001 | |||||
| Lactation first child (months) | ||||||||||||||||
| None | 137 | 65 | 1.12 | 0.77 | - | 1.63 | 0.548 | 104 | 27 | 1.13 | 0.68 | - | 1.88 | 0.646 | ||
| 1–6 | 323 | 134 | 1.00 | 359 | 75 | 1.00 | ||||||||||
| >6 | 139 | 112 | 1.55 | 1.10 | - | 2.18 | 0.012 | 78 | 12 | 0.68 | 0.35 | - | 1.34 | 0.266 | ||
| Six-month trend* | 1.17 | 1.01 | - | 1.34 | 0.031 | 0.82 | 0.59 | - | 1.15 | 0.249 | 0.040 | |||||
| No. of miscarriages | ||||||||||||||||
| None | 584 | 317 | 1.00 | 647 | 132 | 1.00 | ||||||||||
| One or more | 165 | 70 | 0.81 | 0.59 | - | 1.13 | 0.212 | 209 | 43 | 1.09 | 0.74 | - | 1.62 | 0.657 | 0.686 | |
| Age at menarche (years) | ||||||||||||||||
| <12 | 134 | 58 | 0.84 | 0.57 | - | 1.22 | 0.353 | 186 | 45 | 1.51 | 0.98 | - | 2.33 | 0.059 | ||
| 12–13 | 317 | 161 | 1.00 | 432 | 66 | 1.00 | ||||||||||
| >13 | 289 | 166 | 0.99 | 0.75 | - | 1.32 | 0.972 | 234 | 61 | 1.57 | 1.06 | - | 2.34 | 0.026 | ||
| Trend per year* | 1.03 | 0.96 | - | 1.11 | 0.430 | 1.04 | 0.94 | - | 1.16 | 0.444 | 0.861 | |||||
| Hormonal contraception use | ||||||||||||||||
| Never | 475 | 305 | 1.00 | 334 | 85 | 1.00 | ||||||||||
| Ever | 274 | 82 | 0.55 | 0.40 | - | 0.75 | <0.001 | 522 | 90 | 0.75 | 0.53 | - | 1.06 | 0.099 | 0.179 | |
| < = 5 years | 105 | 34 | 0.59 | 0.38 | - | 0.92 | 0.019 | 210 | 41 | 0.82 | 0.53 | - | 1.26 | 0.368 | ||
| >5 years | 78 | 25 | 0.66 | 0.40 | - | 1.10 | 0.111 | 171 | 21 | 0.51 | 0.30 | - | 0.87 | 0.014 | ||
| Not known | 91 | 23 | 0.42 | 0.25 | - | 0.70 | 0.001 | 141 | 28 | 0.94 | 0.57 | - | 1.55 | 0.806 | ||
| Postmenopausal women | ||||||||||||||||
| Age at menopause (years) | ||||||||||||||||
| < = 45 | 142 | 64 | 0.80 | 0.54 | - | 1.20 | 0.283 | 103 | 33 | 1.22 | 0.69 | - | 2.15 | 0.494 | ||
| 46–49 | 143 | 79 | 0.95 | 0.65 | - | 1.39 | 0.787 | 116 | 31 | 1.07 | 0.60 | - | 1.90 | 0.815 | ||
| 50–52 | 164 | 102 | 1.00 | 130 | 33 | 1.00 | ||||||||||
| >52 | 138 | 74 | 0.88 | 0.60 | - | 1.30 | 0.528 | 74 | 20 | 0.91 | 0.48 | - | 1.73 | 0.774 | ||
| Five-year trend* | 1.05 | 0.92 | - | 1.21 | 0.465 | 0.87 | 0.71 | - | 1.06 | 0.161 | 0.114 | |||||
| Fertility time (years) | ||||||||||||||||
| <33 | 137 | 79 | 1.00 | 99 | 30 | 1.00 | ||||||||||
| 33–36 | 162 | 93 | 1.00 | 0.67 | - | 1.48 | 0.992 | 107 | 35 | 1.04 | 0.58 | - | 1.85 | 0.904 | ||
| 37–39 | 144 | 74 | 0.85 | 0.56 | - | 1.28 | 0.428 | 119 | 28 | 0.74 | 0.40 | - | 1.34 | 0.317 | ||
| >39 | 138 | 72 | 0.86 | 0.57 | - | 1.30 | 0.477 | 95 | 23 | 0.68 | 0.36 | - | 1.28 | 0.231 | ||
| Five-year trend* | 1.02 | 0.89 | - | 1.16 | 0.774 | 0.87 | 0.71 | - | 1.06 | 0.168 | 0.189 | |||||
| Hormone therapy use | ||||||||||||||||
| Never | 585 | 350 | 1.00 | 440 | 121 | 1.00 | ||||||||||
| Ever | 65 | 10 | 0.30 | 0.15 | - | 0.60 | 0.001 | 59 | 12 | 0.74 | 0.38 | - | 1.47 | 0.393 | 0.064 | |
a Totals do not add up because of missing values.
b Odds ratios (ORs) and 95% confidence intervals (95% CI) adjusted for age, BMI 1-year prior to the interview, family history of colorectal cancer, tobacco, hormone therapy use and hormonal contraception use (the latter two variables were excluded as confounders when analyzing their association with colorectal cancer risk). Province was included as a random effect term.
c P-int.: P value of the interaction term between educational level and the corresponding variable.
* In italics: ORs, 95% CI and P values obtained with the corresponding variable as a continuous term.
Discussion
This case-control study with population controls examines the association between recalled menstrual and reproductive factors and GC and CRC risk, and evaluates whether the effect differ by educational level. Our results show a decreased GC risk associated with older age at first birth; a decreased GC, CC and RC risk associated with ever use of hormonal contraception and a decreased CC and RC risk among postmenopausal hormone therapy users. Women with low educational level who had more children or who breastfed their first child for longer periods registered an increased CRC risk, while those with higher educational background showed the opposite effect.
One of the main strengths of this study is its large sample size, since to date it is the largest epidemiological study that analyses the association between GC and CRC risk and menstrual and reproductive factors in the Spanish population. The study was carried out in 11 Spanish provinces, covering rural and urban areas. In addition, we have used histologically confirmed incident cases and population-based controls. Finally, the random province-specific intercept term included in our statistical models allowed us to take into account unexplained heterogeneity due to unmeasured factors across different provinces.
Some limitations should also be considered. First, self-reported information is subjected to recall bias. However, if this bias exists, it would probably be non-differential, since the possible association between reproductive factors and GC and CRC risk is largely unknown. Several previous studies have concluded that self-reported information of age at menopause, age at menarche, number of children, age at first pregnancy, age at first and last birth and spontaneous abortions are recalled with reasonable accuracy [18, 19]. These same variables were used in a previous study of our group that analyzed the influence of obstetric factors on mammographic density in adult Spanish women [20]. In a quality control analysis of that study, which included re-test in a subsample of women, we showed acceptable levels of reproducibility. Regarding a potential selection bias, we have evaluated possible differences between women with complete and missing data in terms of demographic and other confounding variables and we observed that women unable to provide this information were older and had lower educational background than those who did, being this difference similar in cases and controls. Another limitation is the high number of missing values in dietary variables. However, when we replicated the analyses introducing these variables in the models (S1 Table and S2 Table) results were not altered. It could be possible that unmeasured confounders affect our findings. Nevertheless, most established GC risk factors were controlled in the present study, with the exception of Helicobacter pylori infection. However, the infection caused by this bacterium is not a confounding factor in our study since, in agreement with the literature, no association was observed between this infection, measured by a serological assay, and the reproductive factors studied here (data not shown). Moreover, those unmeasured characteristics that could have a geographical distribution have been at least partly accounted for through the random effect province term included in our statistical analyses. Finally, we have small sample size to find statistically significant associations when evaluating certain subgroup associations for GC, such as the stratified analyses by anatomic subsite, histologic type or educational level.
Age at first birth was inversely associated with GC risk in the present study. In a previous meta-analysis no association with this variable was detected [11]. Nevertheless, some studies of this meta-analysis have found a borderline inverse association of age at first birth with GC in general [21], with all subsites and histological types of GC [22], with adenocarcinomas of the gastric cardia [23, 24], and with non-cardia gastric cancer in postmenopausal women[23].
Our results suggest a protective role of exogenous female hormones on gastric and colorectal cancer risk. Previous studies of oral contraceptives and GC have reported risk estimates from 0.79 to 2.50, with a pooled relative risk for ever use of 1.11 [11]. Only Frise et al detected a no significant decreased risk of gastric adenocarcinoma, more pronounced for the intestinal histologic subtype [25]. Nevertheless, the protective effect of hormone replacement therapy on GC risk has been reported in several studies [11, 26]. Evidence suggests that estrogens may offer protection against the development and progression of GC by acting on ERα and ERβ. The biological pathway is unclear, but several mechanisms have been suggested, such as the increased expression of trefoil factor genes, the inhibition of oncogenes’ expression or decreasing bile acid concentration [27]. For this reason, it would be reasonable to think that parity might be associated with GC risk through these pathways. However, in consonance with our findings, the most recent meta-analysis of prospective cohort studies found no significant association [28].
With respect to CRC, three previous meta-analyses described an inverse association with oral contraceptives use, with summary relative risks of 0.81 [29] and 0.82 [30, 31]. Two of them detected no differences according to duration of use, although there were indications that the protection was stronger for more recent use [29, 30]. Meanwhile, Luan et al described a statistically significant nonlinear inverse association with duration of use [31]. Regarding hormone replacement therapy, two previous meta-analyses found an approximately 20% reduction in CRC risk among ever users [32, 33], similar for colon and rectal tumors [32]. This protective effect seems to be modulated through the ERβ, the predominant ER in the colon. During the tumorigenesis process, the ERβ expression in colonocytes is lost, and hormone replacement therapy exerts its effects through preventing this loss [8]. Increased local concentration of estrogens reduces the production of carcinogenic secondary bile acid, limits DNA damage and microsatellite instability and inhibits cell proliferation of colonic tumors [9].
When analyzing CRC risk, we have detected an interaction between months of first birth lactation and parity with educational level. Frise et al, in a study of reproductive factors and risk of gastric adenocarcinoma, also detected a statistically significant interaction between income level and parity [25]. With respect to lactation, previous studies have detected no association [34–36], except Lo et al, who found an inverse association with CRC risk [37]. Regarding parity, Guan et al, in a meta-analysis of prospective studies, found a 5% decreased CRC risk among parous versus nulliparous women, although they found no dose-response effect [38]. La Vecchia et al, in 6 out of 18 studies found significant protection by parity on CC or CRC risk [39]. When we additionally adjusted by other dietary CRC risk factors (such as calorie intake, red meat, processed meat, fruits, vegetables, and alcohol consumption) we also observed a significant interaction term with parity (P<0.001) and almost significant with duration of lactation (P = 0.051). Since we do not find a clear biologic rationale for this result, it should be interpreted with caution in terms of prevention. It is possible that we were unable to completely adjust for these imprecisely measured factors, which may have led to some residual confounding. Other lifestyle conditions not yet identified could have influenced on these associations, as well as early-life conditions that could not be controlled for, given that these women grew up in a tough era marked by the Spanish civil war and long postwar period.
In brief, our results provide some support for the hypothesis that oral contraceptives and hormone replacement therapy decrease GC and CRC risk in women. We detected a decreased GC risk associated with advanced maternal age at first birth and a significant interaction of educational level with parity and breastfeeding. These findings are consistent with the exogenous hormone hypothesis. However, the role of endogenous hormones on GC and CRC risk remains to be elucidated, and should be explored taking into account socioeconomic factors and lifestyles closely related with reproductive patterns.
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
The authors are sincerely grateful to the participants and staff of the collaborating hospitals and primary care centers and to other investigators of the MCC-Spain Project for their contributions to this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by the Consortium for Biomedical Research in Epidemiology and Public Health (CIBERESP), by the “Acción Transversal del Cáncer," approved on the Spanish Ministry Council on the 11th October 2007, by the Instituto de Salud Carlos III grants PI08/1770 (to M. Kogevinas), PI09/0773 (to J. LLorca), PI09/1286 (to V. Martín), PI09/1903 (to R. Peiró), PI09/1662 (to J.J. Jiménez-Moleón), PI11/01403 (to N. Aragonés), by the Instituto de Salud Carlos III, co-funded by FEDER funds – a way to build Europe – PI08-1359, PI14-0613 (to V. Moreno), by the European Commission grants FOOD-CT-2006-036224-HIWATE (to V. Moreno), by the Catalan Government DURSI grant 2014SGR647 (to V. Moreno), by the Fundación Marqués de Valdecilla grant API 10/09 (to J. Llorca), by the Junta de Castilla y León grant LE22A10-2 (to V. Martín), by the Consejería de Salud of the Junta de Andalucía grant 2009-S0143 (to J. Alguacil), and by the Conselleria de Sanitat of the Generalitat Valenciana grant AP061/10 (to R. Peiró). The funders had no role in the study design and data analysis.
References
- 1.Galceran J, Ameijide A, Carulla M, Mateos A, Quirós JR, Alem n A, et al. Estimaciones de la incidencia y la supervivencia del cáncer en España y su situación en Europa Red Española de Registros de Cáncer (REDECAN) 2014. [Google Scholar]
- 2.Mortalidad por cáncer y otras causas en España. 2013 [Internet]. National Center for Epidemiology (NCE), Carlos III Institute of Health. 2016. Available: http://www.isciii.es/ISCIII/es/contenidos/fd-servicios-cientifico-tecnicos/fd-vigilancias-alertas/fd-epidemiologia-ambiental-y-cancer/Mortal_2013.pdf.
- 3.Colorectal (CRC)—Large Bowel Cancer Factsheet [Internet]. European Network of Cancer Registries (ENCR). 2013. Available: http://encr.eu/images/docs/factsheets/ENCR_Factsheet_Colorectal_2013.pdf.
- 4.Lopez-Abente G, Ardanaz E, Torrella-Ramos A, Mateos A, Delgado-Sanz C, Chirlaque MD. Changes in colorectal cancer incidence and mortality trends in Spain. AnnOncol. 2010;21 Suppl 3:iii76–iii82. [DOI] [PubMed] [Google Scholar]
- 5.Interactive epidemiological information system (ARIADNA) [Internet]. National Center for Epidemiology (NCE), Carlos III Institute of Health. 2016. Available: http://ariadna.cne.isciii.es/evindex.html.
- 6.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. EurJCancer. 2013;49(6):1374–403. [DOI] [PubMed] [Google Scholar]
- 7.Chandanos E, Rubio CA, Lindblad M, Jia C, Tsolakis AV, Warner M, et al. Endogenous estrogen exposure in relation to distribution of histological type and estrogen receptors in gastric adenocarcinoma. GastricCancer. 2008;11(3):168–74. [DOI] [PubMed] [Google Scholar]
- 8.Barzi A, Lenz AM, Labonte MJ, Lenz HJ. Molecular pathways: Estrogen pathway in colorectal cancer. ClinCancer Res. 2013;19(21):5842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster PA. Oestrogen and colorectal cancer: mechanisms and controversies. Int J Colorectal Dis. 2013;28(6):737–49. 10.1007/s00384-012-1628-y [DOI] [PubMed] [Google Scholar]
- 10.Zervoudakis A, Strickler HD, Park Y, Xue X, Hollenbeck A, Schatzkin A, et al. Reproductive history and risk of colorectal cancer in postmenopausal women. J Natl Cancer Inst. 2011;103(10):826–34. 10.1093/jnci/djr101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(1):20–38. 10.1158/1055-9965.EPI-11-0834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duell EJ, Travier N, Lujan-Barroso L, Boutron-Ruault MC, Clavel-Chapelon F, Palli D, et al. Menstrual and reproductive factors, exogenous hormone use, and gastric cancer risk in a cohort of women from the European Prospective Investigation Into Cancer and Nutrition. Am J Epidemiol. 2010;172(12):1384–93. 10.1093/aje/kwq321 [DOI] [PubMed] [Google Scholar]
- 13.Costas L, Sequera VG, Quesada P, Altzibar JM, Lope V, Perez-Gomez B, et al. Hormonal contraception and postmenopausal hormone therapy in Spain: time trends and patterns of use. Menopause. 2015;22(10):1138–46. 10.1097/GME.0000000000000487 [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Arenzana N, Navarrete-Munoz EM, Peris M, Salas D, Ascunce N, Gonzalez I, et al. Diet quality and related factors among Spanish female participants in breast cancer screening programs. Menopause. 2012;19(10):1121–9. 10.1097/gme.0b013e3182544925 [DOI] [PubMed] [Google Scholar]
- 15.Peiro-Perez R, Salas D, Valles G, Abad-Fernandez MS, Vidal C, Sanchez-Contador EC, et al. Walking, biking or sport: how Spanish women attending breast cancer screening meet physical activity recommendations? EurJPublic Health. 2015;25(5):857–63. [DOI] [PubMed] [Google Scholar]
- 16.Castano-Vinyals G, Aragones N, Perez-Gomez B, Martin V, Llorca J, Moreno V, et al. Population-based multicase-control study in common tumors in Spain (MCC-Spain): rationale and study design. GacSanit. 2015;29(4):308–15. [DOI] [PubMed] [Google Scholar]
- 17.MCC-Spain Study. Questionnaire. Eighteenth version [updated March 2, 2010. Available: http://www.mccspain.org/sites/default/files/Quest_MCCSpain.pdf.
- 18.Bosetti C, Tavani A, Negri E, Trichopoulos D, La VC. Reliability of data on medical conditions, menstrual and reproductive history provided by hospital controls. JClinEpidemiol. 2001;54(9):902–6. [DOI] [PubMed] [Google Scholar]
- 19.Cairns BJ, Liu B, Clennell S, Cooper R, Reeves GK, Beral V, et al. Lifetime body size and reproductive factors: comparisons of data recorded prospectively with self reports in middle age. BMCMedResMethodol. 2011;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lope V, Perez-Gomez B, Sanchez-Contador C, Santamarina MC, Moreo P, Vidal C, et al. Obstetric history and mammographic density: a population-based cross-sectional study in Spain (DDM-Spain). Breast Cancer Res Treat. 2012;132(3):1137–46. 10.1007/s10549-011-1936-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman ND, Chow WH, Gao YT, Shu XO, Ji BT, Yang G, et al. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut. 2007;56(12):1671–7. 10.1136/gut.2007.129411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue M, Ito LS, Tajima K, Yamamura Y, Kodera Y, Takezaki T, et al. Height, weight, menstrual and reproductive factors and risk of gastric cancer among Japanese postmenopausal women: analysis by subsite and histologic subtype. Int J Cancer. 2002;97(6):833–8. [DOI] [PubMed] [Google Scholar]
- 23.Bahmanyar S, Lambe M, Zendehdel K, Nyren O, Boffetta P, Ye W. Parity and risk of stomach cancer by sub-site: a national Swedish study. BrJ Cancer. 2008;98(7):1295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedman ND, Lacey JV Jr., Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. The association of menstrual and reproductive factors with upper gastrointestinal tract cancers in the NIH-AARP cohort. Cancer. 2010;116(6):1572–81. 10.1002/cncr.24880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frise S, Kreiger N, Gallinger S, Tomlinson G, Cotterchio M. Menstrual and reproductive risk factors and risk for gastric adenocarcinoma in women: findings from the canadian national enhanced cancer surveillance system. Ann Epidemiol. 2006;16(12):908–16. 10.1016/j.annepidem.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 26.Green J, Czanner G, Reeves G, Watson J, Wise L, Roddam A, et al. Menopausal hormone therapy and risk of gastrointestinal cancer: nested case-control study within a prospective cohort, and meta-analysis. IntJCancer. 2012;130(10):2387–96. [DOI] [PubMed] [Google Scholar]
- 27.Chandanos E, Lagergren J. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer. 2008;44(16):2397–403. 10.1016/j.ejca.2008.07.031 [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Gong TT, Wu QJ. Parity and gastric cancer risk: a systematic review and dose-response meta-analysis of prospective cohort studies. Sci Rep. 2016;6:18766 10.1038/srep18766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosetti C, Bravi F, Negri E, La VC. Oral contraceptives and colorectal cancer risk: a systematic review and meta-analysis. HumReprodUpdate. 2009;15(5):489–98. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez E, La VC, Balducci A, Chatenoud L, Franceschi S, Negri E. Oral contraceptives and colorectal cancer risk: a meta-analysis. BrJCancer. 2001;84(5):722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luan NN, Wu L, Gong TT, Wang YL, Lin B, Wu QJ. Nonlinear reduction in risk for colorectal cancer by oral contraceptive use: a meta-analysis of epidemiological studies. Cancer Causes Control. 2015;26(1):65–78. 10.1007/s10552-014-0483-2 [DOI] [PubMed] [Google Scholar]
- 32.Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. AmJMed. 1999;106(5):574–82. [DOI] [PubMed] [Google Scholar]
- 33.Lin KJ, Cheung WY, Lai JY, Giovannucci EL. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. IntJCancer. 2012;130(2):419–30. [DOI] [PubMed] [Google Scholar]
- 34.Akhter M, Inoue M, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S. Reproductive factors, exogenous female hormone use and colorectal cancer risk: the Japan Public Health Center-based Prospective Study. Eur J Cancer Prev. 2008;17(6):515–24. 10.1097/CEJ.0b013e3282f521f8 [DOI] [PubMed] [Google Scholar]
- 35.Jacobsen BK, Vollset SE, Kvale G. Do reproductive factors influence colorectal cancer survival? J Clin Epidemiol. 1995;48(9):1119–22. [DOI] [PubMed] [Google Scholar]
- 36.Tsilidis KK, Allen NE, Key TJ, Bakken K, Lund E, Berrino F, et al. Oral contraceptives, reproductive history and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition. BrJ Cancer. 2010;103(11):1755–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo AC, Soliman AS, Khaled HM, Aboelyazid A, Greenson JK. Lifestyle, occupational, and reproductive factors and risk of colorectal cancer. Dis Colon Rectum. 2010;53(5):830–7. 10.1007/DCR.0b013e3181d320b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan HB, Wu QJ, Gong TT, Lin B, Wang YL, Liu CX. Parity and risk of colorectal cancer: a dose-response meta-analysis of prospective studies. PLoSOne. 2013;8(9):e75279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.La Vecchia C, Franceschi S. Reproductive factors and colorectal cancer. Cancer Causes Control. 1991;2(3):193–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.