Abstract
Glycosaminoglycans (GAGs) such as heparin are promising materials for growth factor delivery due to their ability to efficiently bind positively charged growth factors including bone morphogenetic protein-2 (BMP-2) through their negatively charged sulfate groups. Therefore, the goal of this study was to examine BMP-2 release from heparin-based microparticles (MPs) after first, incorporating a hydrolytically degradable crosslinker and varying heparin content within MPs to alter MP degradation and second, altering the sulfation pattern of heparin within MPs to vary BMP-2 binding and release. Using varied MP formulations, it was found that the time course of MP degradation for 1 wt% heparin MPs was ~4 days slower than 10 wt% heparin MPs, indicating that MP degradation was dependent on heparin content. After incubating 100 ng BMP-2 with 0.1 mg MPs, most MP formulations loaded BMP-2 with ~50% efficiency and significantly more BMP-2 release (60% of loaded BMP-2) was observed from more sulfated heparin MPs (MPs with ~100% and 80% of native sulfation). Similarly, BMP-2 bioactivity in more sulfated heparin MP groups was at least four-fold higher than soluble BMP-2 and less sulfated heparin MP groups, as determined by an established C2C12 cell alkaline phosphatase (ALP) assay. Ultimately, the two most sulfated 10 wt% heparin MP formulations were able to efficiently load and release BMP-2 while enhancing BMP-2 bioactivity, making them promising candidates for future growth factor delivery applications.
Introduction
Bone morphogenetic protein-2 (BMP-2) is an osteoinductive growth factor that is FDA-approved for use in specific orthopaedic procedures including spinal fusions and tibial fracture repairs1,2. Currently supraphysiological levels of BMP-2 are required to induce bone repair, primarily due to the inability of biomaterial delivery vehicles to maintain growth factor bioactivity or spatiotemporally control growth factor release3,4. These high and costly doses of BMP-2 ultimately result in undesirable side effects, including inflammation and bone formation with cyst-like voids or poor mechanical properties5,6. Therefore, a novel strategy to maintain BMP-2 bioactivity while releasing it in a controlled manner is in high demand.
Previous efforts to fabricate BMP-2 delivery vehicles have employed alginate, chitosan, collagen, gelatin, and synthetic polymers derived from polyethylene glycol (PEG) or poly(lactic acid)7–11. However, we and other laboratories12–16 are exploring the use of glycosaminoglycans (GAGs) to potentially reduce burst release of BMP-2 and prolong growth factor bioactivity9,11,17. GAGs are linear polysaccharides found within the extracellular matrix (ECM) either as free chains or, more often, covalently bound to a polypeptide core, collectively known as proteoglycans18,19. GAGs can bind growth factor through carbohydrate sequence-specific interactions and sulfated GAGs can also bind positively-charged growth factors via their negatively-charged sulfate groups19. Therefore, sulfation pattern can be adjusted experimentally to further alter the binding and release of growth factor from GAG molecules20–23. Ultimately, GAGs can immobilize growth factors near cells and prevent growth factor degradation, making GAGs a promising biomaterial for growth factor delivery18,24,25.
Heparin is a highly sulfated GAG species found mainly in mast cells, although heparan sulfate, a similar GAG species, is abundantly found on cell surfaces throughout the body26. Heparin is capable of non-covalent and reversible binding to a wide variety of positively charged growth factors including BMP-2, transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), fibroblast growth factors (FGFs) and insulin-like growth factors (IGFs)19,25,27. Our laboratory and others have incorporated heparin into bulk hydrogels for improved BMP-2 loading and release12–16, but microparticles (MPs) are advantageous to efficiently load growth factors with little excess material due to the MP’s high surface area-to-volume ratio9,28. Therefore, microgels and MPs with heparin have also been fabricated as BMP-2 carriers29–31, and we have demonstrated that 100 wt% heparin MPs can bind significant amounts of BMP-2 with up to 95–100% loading efficiency and enhanced BMP-2 bioactivity32.
The potential drawback of including large amounts of heparin in MPs, however, is that little BMP-2 release was observed from 100% heparin MPs due to sequestration of growth factor, limiting the efficiency of these MPs as soluble growth factor delivery vehicles30,32. Therefore, in this study we explored two methods to facilitate more complete growth factor release from MPs while maintaining their ability to bind and preserve growth factor bioactivity. First, by incorporating a hydrolytically degradable crosslinker and varying heparin content within heparin-based MPs, we hypothesized that the release of growth factor could be enhanced as the MP degrades. Secondly, given that heparin sulfation is primarily responsible for electrostatic growth factor-GAG binding and release, we hypothesized that systematically reducing the degree of heparin sulfation may promote the release of BMP-2 from heparin MPs. Furthermore, since desulfated GAGs have been shown to have diminished anti-coagulation properties, desulfated heparin MPs may be safer for future in vivo therapies18,22,33. Therefore, we fabricated a series of MPs with selectively desulfated heparin derivatives to further control BMP-2 release. Overall, we hypothesized that altering the degradation properties and sulfation pattern of heparin MPs, two orthogonal approaches to control BMP-2 release from MPs, would allow for tunable growth factor delivery kinetics from these materials.
Experimental
Preparation of heparin derivatives
Preparation of desulfated Hep−N, Hep−6O,N, and Hep- species was carried out as described previously34. Briefly, heparin sodium salt from porcine intestinal mucosa (Sigma Aldrich, St.Louis, MO) was reconstituted at ~10 mg/mL in water and passed through Dowex 50WX4 resin (mesh size 100–200, Sigma Aldrich). Pyridine was added drop-wise to the desalted heparin until the pH of the solution was ~6.0. Excess water and pyridine were removed on a rotary evaporator (Buchi). The heparin pyridinium salt solution was flash frozen in liquid nitrogen, lyophilized to a powder, and stored at −20°C.
For Hep−N preparation, heparin pyridinium salt was dissolved at 1 mg/mL in 90% DMSO/10% water (v/v) and mixed at 50°C for 2 hours35,36. For Hep−6O,N, heparin pyridine was dissolved at 10 mg/mL in 90% N-methylpyrrolidone (NMP, Acros Organics, Belgium)/10% water (v/v) and maintained at 90°C for 48 hours20. Hep- was prepared under identical conditions to Hep−6O,N but the reaction proceeded at 100°C for 24 hours. Following each reaction, each heparin solution was cooled on ice and precipitated with 95% ethanol saturated with sodium acetate. The heparin precipitates were stirred for 2 hours on ice and centrifuged to remove excess ethanol and water. The resulting material was dissolved in water, dialyzed for 3 days, lyophilized to a powder, and stored at −20°C.
Thiolation of heparin derivatives
400 mg of each heparin derivative was dissolved in water with 0.2–4.0 molar excess hydroxybenzotriazole (HOBt, Ark Pharm, Libertyville, IL) and cystamine. After adjusting the pH to 5.0, 0.2–4.0 molar excess 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC, Sigma Aldrich) was added and the reaction was allowed to proceed for 2 hours (see Table 1). Following the reaction, the solution was dialyzed for 3 days, lyophilized to a powder, and stored at −20°C37,38.
Table 1.
Molar excesses of reagents used for thiolation of heparin derivatives.
| Heparin species | EDC [x molar] | HOBt [x molar] | Cystamine [x molar] |
|---|---|---|---|
| Hep | 0.2 | 0.2 | 0.2 |
| Hep−N | 1.0 | 1.0 | 1.0 |
| Hep−6O,N | 2.0 | 2.0 | 2.0 |
| Hep- | 4.0 | 4.0 | 2.0 |
Material characterization
Proton nuclear magnetic resonance (1H NMR) was performed to determine the degree of thiolation and sulfation of the various chemically modified heparin species. Heparin derivatives were dissolved in deuterated water (10 mg/mL) and 1H NMR spectra were recorded on a Bruker Avance III 400 spectrometer at 400 MHz as described previously34.
Microparticle fabrication and size analysis
To functionalize 4-arm polyethylene glycol (PEG, 10 kDa, Creative PEGWorks, Chapel Hill, NC), PEG was reacted with acryloyl chloride (AcCl, Sigma Aldrich) in an 8:1 AcCl to PEG molar ratio in dichloromethane (DCM) solution39. Triethylamine (TEA, Sigma Aldrich) was added drop-wise in a 1:1 TEA to AcCl molar ratio as a catalyst to yield 4-arm PEG acrylate (PEG-4Ac). To prepare the aqueous phase for 10 wt% heparin MPs, 5.6 mg thiolated heparin, 50 mg PEG-4Ac, and 16 μL 50 mM tris-(2-carboxyethyl)-phosphine (TCEP, pH 9, Calbiochem, Germany) were dissolved in 200 μL water. For 1 wt% heparin MPs, 0.6 mg thiolated heparin, 55 mg PEG-4Ac, and 16 μL 50 mM TCEP were dissolved in 220 μL water. After all heparin/PEG-4Ac solutions incubated for 15 minutes at 37°C, water (pH 3) and 25 mM D,L-dithiothreitol (DTT, Sigma Aldrich) were added to yield a final aqueous phase volume of 320 μL.
A homogenizer (Polytron PT 3100, Kinematica, Bohemia, NY) was set to 3,100 RPM and a water bath set to 26°C was configured underneath the homogenizer probe. The oil phase, 15 mL mineral oil (Amresco, Solon, OH) + 0.05% v/v Span 80 (TCI, Cambridge, MA), was placed under the homogenizer and the aqueous phase was subsequently added via pipette. After 2 minutes, 5 mL mineral oil containing between 5–13 μL TEA was added to the emulsion via syringe and homogenized for 10 minutes (Table 2).
Table 2.
TEA amounts added for each MP formulation.
| 10 wt% heparin MPs | TEA (μL) | 1 wt% heparin MPs | TEA (μL) |
|---|---|---|---|
| Hep | 13.0 | Hep | 8.0 |
| Hep−N | 12.0 | Hep−N | 7.4 |
| Hep−6O,N | 9.5 | Hep−6O,N | 5.2 |
| Hep- | 11.1 | Hep- | 6.8 |
After homogenizing, 25 mL 0.8 mM Pluronic F 127 (Pluronic buffer, Sigma Aldrich) + 0.5% v/v acetic acid (VWR) was added to the emulsion and this solution was centrifuged at 4100 RPM for 10 minutes. The oil phase was removed, MPs were washed with Pluronic buffer two additional times and then stored at 4°C. MP average size and distribution analysis were performed on newly formed MPs via phase contrast imaging (Nikon i80 microscope), with Image J and Origin 9 software. A minimum of 150 MPs were measured per experimental group.
Microparticle degradation
MP degradation was monitored by incubating 0.1 mg of all MP formulations in 0.5 mL 0.5% v/v BSA (Thermo Scientific, Norcross, GA) + 0.5% NaN3 (Alfa Aesar, Ward Hill, MA) phosphate buffered saline (PBS) solution at 37°C. To capture MP degradation over time, 30 μL of each sample was removed and imaged via phase microscopy at day 1, 2, 4, 7, 10, and 14. Approximately 25 MPs were captured per image at Day 1 and a minimum of 12 images were taken per group at each time point.
BMP-2 loading and release
Recombinant human BMP-2 (R&D Systems, Minneapolis, MN) was dissolved at 100 μg/mL in sterile water and single use aliquots were frozen until use. For loading and release studies, 0.1 mg of each MP formulation and 100 ng BMP-2 were incubated in 0.5 mL 0.5% BSA PBS solution for 16 h at 4°C. The amount of BMP-2 was chosen to ensure that the concentration of BMP-2 released from the MPs falls within the linear range of the BMP-2 bioactivity assay. After 16 hours, MPs were centrifuged at 15,000 RCF for 3 min, the supernatant was removed, and MPs were re-suspended in 0.5 mL fresh 0.5% BSA solution. The supernatant removed at 16 and 19 h was used to quantify loading onto MPs via BMP-2 ELISA (R&D Systems). Assuming that BMP-2 released over the initial 3 h was not specifically bound to the MPs, the BMP-2 in the supernatant at 16 and 19 h was subtracted from the amount of BMP-2 in BMP-2 samples that had been incubated for the same time without MPs to determine the amount of BMP-2 bound/loaded onto MPs. Following this loading procedure, MPs were incubated at 37°C and supernatant was collected on days 1, 2, 4, 7, and 10 to quantify BMP-2 release (n = 3–5).
BMP-2 bioactivity after microparticle loading and release
Using an established cell-based BMP-2 bioactivity assay, 1.92×104 C2C12 myoblasts/cm2 (ATCC) were plated into 96-well plates with 100 μL media consisting of 4.5 g/mL glucose Dulbecco’s Modified Eagle Medium (DMEM, Cellgro, Manassas, VA), 10% v/v fetal bovine serum (FBS, Atlanta Biologics, Atlanta, GA), 1% v/v 10000 IU penicillin/10000 μg/mL streptomycin (Mediatech, Manassas, VA), and 1% v/v 200 mM L-glutamine (Cellgro)40. After 6 hours of attachment, cells were treated with media only, 75 ng soluble BMP-2, 0.1 mg unloaded 10% Hep MPs, or 0.1 mg of all MP formulations loaded with BMP-2. After MPs were loaded with BMP-2 as described above, all groups were maintained for 3 days at 37°C and 95% O2, 5% CO2, after which cells were lysed with 100 μL lysis buffer for alkaline phosphatase (ALP) activity and double stranded DNA (dsDNA) quantification.
ALP activity in C2C12 cells was assessed via production of p-nitrophenol (Sigma Aldrich). All lysed cell samples were frozen, thawed and sonicated three times to completely dissociate the cells. 20 μL of sample or p-nitrophenol standards was combined with 5 μL 1.5 M 2-amino-2-methyl-1-propanol (pH 10.25, Sigma Aldrich) in each well of a 96-well plate. Then, 100 μL of a 1:1 mixture 20 mM p-nitrophenol phosphate disodium salt hexahydrate (Sigma Aldrich) and 10 mM MgCl2 was added to each well. All samples and standards were incubated for 1 hour at 37°C, at which point the reaction was terminated by adding 100 μL 1 M NaOH and absorbance was read at 405 nm (n = 3–5). The ALP activity (nmol of p-nitrophenol/mL/min) of each sample was normalized to its respective dsDNA concentration (μg/mL, Supplemental Figure 1).
Statistical analysis
All data are presented as mean ± standard deviation. One-way analysis of variance (ANOVA) and Tukey’s post hoc multiple comparison test with a significance value set at p ≤ 0.05 were used to identify significant differences. Statistical analysis was performed with Minitab (v15.1).
Results
Heparin MP fabrication and characterization
Prior to MP fabrication, four heparin derivatives were prepared through desulfation, including fully sulfated (Hep), N-desulfated (Hep−N), 6O,N-desulfated (Hep−6O,N), and fully desulfated (Hep-) heparin. Total sulfation was quantified via 1H NMR for each heparin derivative and ranged from 0% for Hep- to 100% for Hep (Table 3). Additionally, 1H NMR indicated that all heparin derivatives were successfully thiolated with 10–14% thiolation per disaccharide unit (Table 1). 10 wt% heparin and 1 wt% heparin MPs were formed with each heparin derivative and based upon phase contrast imaging, most MP formulations appeared similar in size, transparency and spherical morphology (Figure 2). Size distribution analysis indicated that a majority of MPs in all formulations were less than 20 μm in diameter and specifically for 1% Hep−N MPs, most were less than 10 μm in diameter (Figure 2Aii). The average diameter of all MPs ranged between 11–14 μm and 1% Hep−N MPs were only significantly smaller than 10% Hep−6O,N MPs.
Table 3.
Sulfation and thiolation degrees of synthesized heparin derivatives.
| Heparin species | Total sulfation [%] | Thiolation [%] per disaccharide units |
|---|---|---|
| Hep | 100 ± 1 | 14 ± 2 |
| Hep-N | 87 ± 2 | 14 ± 2 |
| Hep−6O,N | 20 ± 2 | 10 ± 1 |
| Hep- | 0 ± 0 | 12 ± 2 |
Figure 2.
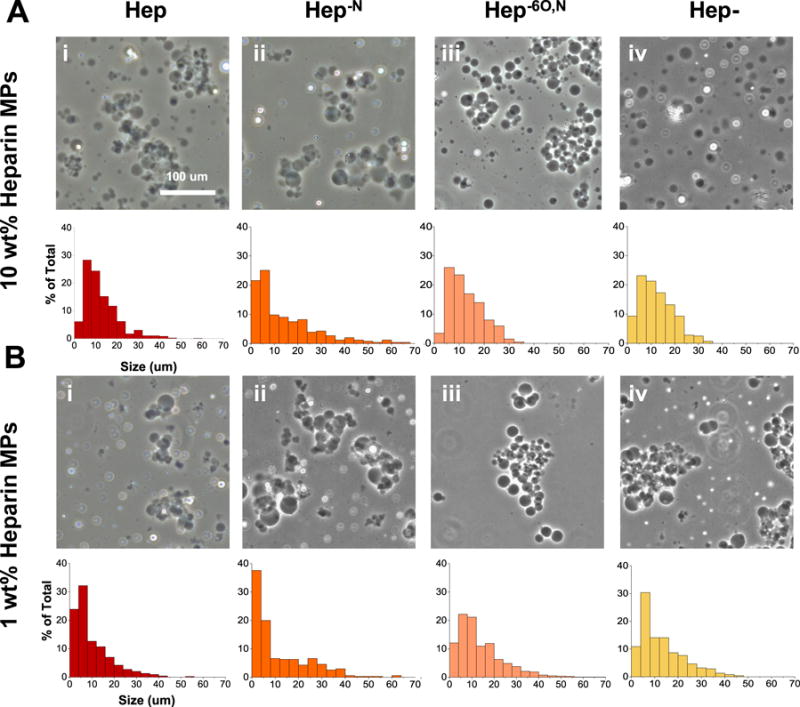
Heparin microparticle size distribution. microparticles contain either 10 wt% heparin (a) or 1 wt% heparin (b) of differing sulfation patterns (i–iv). scale bar is 100 μm.
Heparin MP degradation
In the MP degradation study, all MP formulations remained present at day 4 (Figure 3). By day 7, 10% Hep MPs had degraded and all other 10% heparin MPs appeared to have swelled. Finally by day 10, all remaining 10% heparin MP formulations had degraded. In contrast, though 1% Hep- MPs degraded by day 10, all other 1% heparin MP formulations degraded by day 14 (Table 3). Throughout the degradation process, based on the phase-contrast microscopy images, all MPs appeared to remain similar in shape over time.
Figure 3.
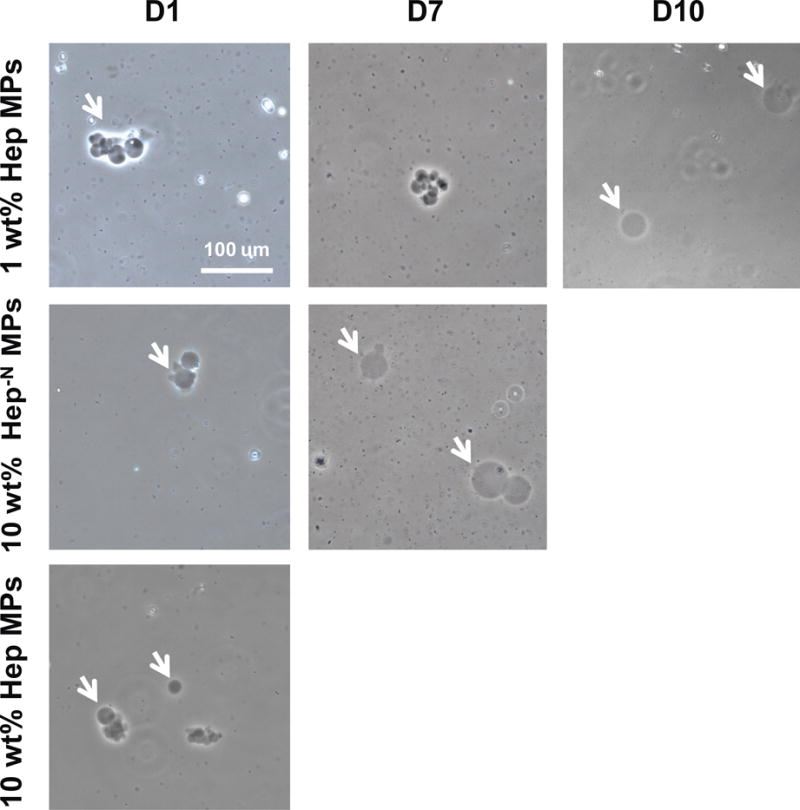
Microparticle degradation time course. First row shows representative images that were similar for 1 wt% hep, 1% hep−n and 1% hep−6on microparticles. second row shows representative images that were similar for 10 wt% hep−n, 10% hep−6on, 10% hep- microparticles, and 1% hep- microparticles. White arrows indicate microparticles and scale bar is 100 μm.
BMP-2 loading and release from MPs
All MP formulations loaded 46–50% of the 100 ng BMP-2, except 1% Hep- MPs, which loaded 56% (Figure 4A). Furthermore, all heparin MPs loaded significantly more BMP-2 than 100% PEG MPs. BMP-2 release from MPs was monitored over 10 days and found to be significantly different dependent on heparin sulfation pattern and amount of heparin in MPs. 10% and 1% heparin MPs with more sulfated heparin derivatives, Hep and Hep−N, released significantly more BMP-2 (at least five-fold) than 10% and 1% heparin MPs with Hep−6O,N and Hep- (Figure 4B–C). Moreover, the 10% Hep and 10% Hep−N MPs released significantly more BMP-2 than 1% Hep and 1% Hep−N MPs. However, similar release kinetics were observed from all MP formulations. More than 95% of the cumulative release occurred between days 1–4 for all MPs, and after day 7 no detectable levels of additional BMP-2 were observed in any MP group.
Figure 4.
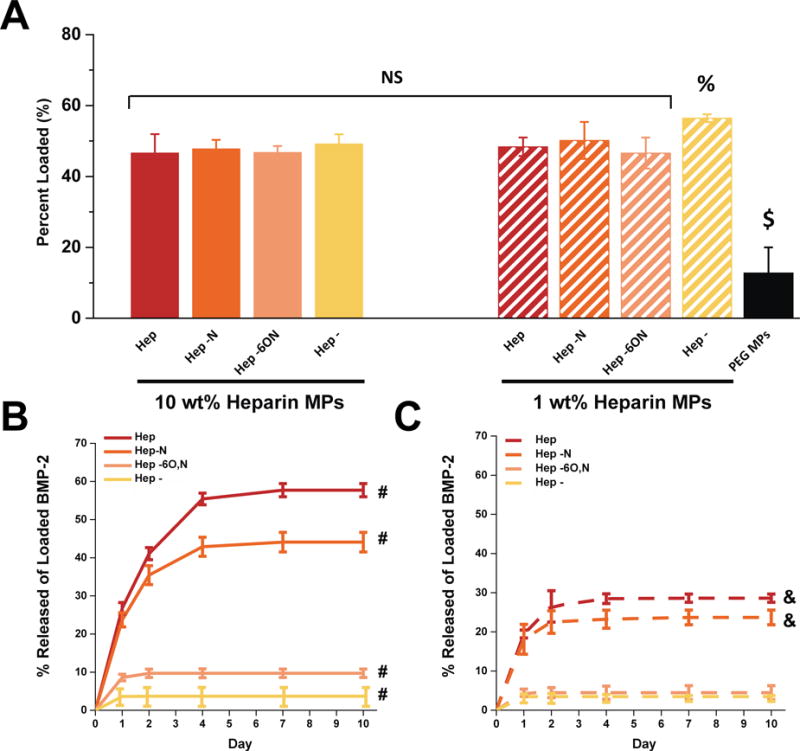
BMP-2 loading and release from all microparticle formulations. (a) BMP-2 loading was similar for most microparticle formulations whereas (b–c) BMP-2 release was significantly higher for more sulfated heparin microparticles with higher heparin content. $significantly different than all other groups; p ≤ 0.05.% significantly different than 10 wt% hep, 10% hep−6o,n, and 1% hep−6o,n microparticle groups; p ≤ 0.05. #All groups significantly different from each other; p ≤ 0.05. & significantly different from 1% hep−6o,n and 1% hep- microparticles; p ≤ 0.05; n = 3–5.
Cell-Based BMP-2 bioactivity assay
For these experiments, the ALP activity of each group was normalized to its dsDNA content (Figure S1). After 3 days, BMP-2-loaded 10% Hep and 10% Hep−N MPs stimulated significantly more ALP activity than all other groups, including control groups of unloaded 10% Hep MPs (no observable ALP activity; data not shown) and 75 ng soluble BMP-2 (Figure 5). Moreover, since the release studies indicated that 10% Hep and 10% Hep−N MPs released less than 20 ng BMP-2 of the ~50 ng loaded over 3 days, these results suggested that BMP-2 bioactivity was enhanced compared to soluble BMP-2. Similarly, 1% Hep and 1% Hep−N MPs stimulated comparable levels of ALP activity to 75 ng soluble BMP-2, though the release studies indicated that these MPs had released less than 10 ng BMP-2 over 3 days. In contrast, the more desulfated heparin MPs, including Hep−6O,N and Hep- MPs, stimulated little to no ALP activity over the 3 day time period regardless of heparin content (10% or 1%).
Figure 5.
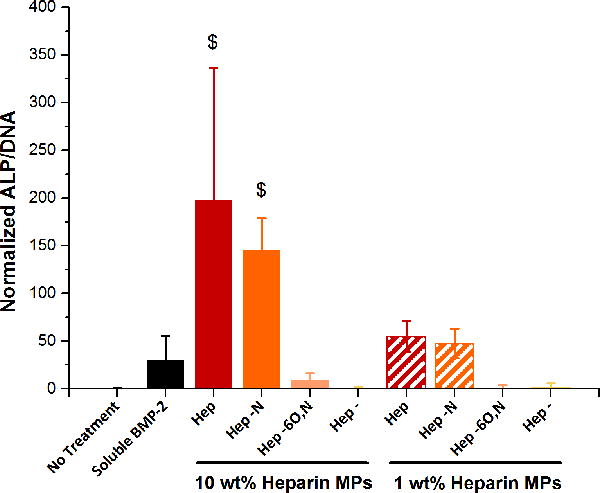
c2c12 alp assay for BMP-2 bioactivity. BMP-2 bioactivity is significantly enhanced in the more sulfated heparin microparticle groups. $significantly different than all other groups; p ≤ 0.05; n = 3–5.
Discussion
In these studies, a series of heparin-based MPs were fabricated for the purpose of controlling release of bioactive BMP-2. In future in vivo applications we envision that these MPs could be combined with gels or mesh-like carriers as an effective orthopaedic therapy. Towards this goal, we investigated (1) the incorporation of hydrolytically degradable crosslinker and alteration of heparin content in MPs to vary MP degradation and (2) the desulfation of heparin within MPs to vary the release of BMP-2 from MPs. Though we have previously developed 100 wt% heparin MPs, to our knowledge the incorporation of degradable crosslinker and desulfated heparin into MPs to further control BMP-2 release has yet to be investigated. Furthermore, the use of Michael Type addition crosslinking within MPs is a novel approach and allows for controlled crosslinking by tuning pH (Figure 1). We deliberately chose to incorporate heparin within PEG MPs because the PEG could be functionalized to include a degradable crosslinker, as well as the fact that it is relatively inert41. Altogether, we examined eight different MP formulations, including 10 wt% heparin and 1 wt% heparin MPs with four heparin species.
Figure 1.
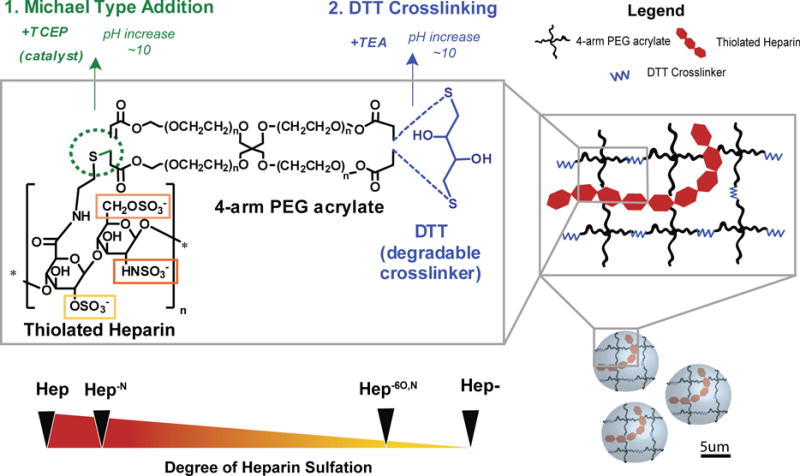
Schematic of microparticle fabrication with thiolated heparin, 4-arm peg acrylate, and dtt crosslinker.
After fabrication, all MPs exhibited spherical morphology, little MP aggregation, and similar size distributions (Figure 2). Overall, the size of our MPs were similar to those made previously with 100 wt% heparin32 to facilitate direct comparisons of growth factor loading and release. To vary MP degradation, we initially sought to modify the DTT concentration in MPs, but found that MPs only formed within a small range of DTT concentrations (15–25 mM DTT) and MP degradation was not significantly altered by DTT content. Instead, we modified the heparin content in MPs and found that 10% heparin MPs degraded sooner than most 1% heparin MPs (Figure 3). With 10-fold less negatively charged heparin within 1% heparin MPs, this may have resulted in less water attraction into the MPs and subsequently slower hydrolysis of the MPs. Similarly, other studies have found that with increasing sulfation, the hydrolytic degradation of sulfated cellulose fibers occurred at lower temperatures, suggesting that more negatively charged materials are increasingly susceptible to hydrolytic degradation42. Finally, it was observed that the 10% Hep MPs were first to degrade of the 10% heparin MPs whereas 1% Hep- MPs were the first to degrade of the 1% heparin MPs. The faster degradation of 1% Hep- MPs may indicate that these MPs were not as stable or did not crosslink as well, and therefore degraded more quickly. Ultimately, we have demonstrated that by reducing the heparin content within MPs the time course of MP degradation was increased by ~4 days for most formulations, thus providing a means to modulate the degradation time of these materials.
In order to produce degradable MPs, the heparin content was reduced compared to the 100 wt% heparin MPs fabricated previously in our laboratory32. However, even with significantly reduced heparin content, most MP formulations exhibited a loading efficiency of ~50% (Figure 4A). Based on these results and previous data from our laboratory, 100%, 10%, and 1% heparin MPs loaded 90, 500, and 5000 ng BMP-2 per 0.1 mg heparin, respectively, though the efficiency of loading can vary depending upon the amounts of growth factor and MPs used32. These differences in growth factor-to-heparin loading efficiency may result from the increasing availability of heparin as the heparin content within MPs is reduced. For 100% heparin MPs, the high density of heparin binding sites may sterically hinder some sites from being occupied, whereas within 10% and 1% heparin MPs, the increased distance between heparin binding sites could potentially allow for more efficient BMP-2 binding. Previous studies in our laboratory found that positively charged protein penetration into heparin-based hydrogels decreased with increasing heparin content, again supporting the idea that MPs with less heparin could load growth factors more efficiently than 100% heparin MPs16. Taken together, these results suggest that reduced heparin content within MPs is still adequate for efficient BMP-2 loading.
Over 10 days, nearly 60% of loaded BMP-2 was released from 10% Hep MPs, substantially more than 100% Hep MPs, which released only 20% in previous studies from our laboratory (Figure 4B)32. In our system, it is possible that the 100% Hep MPs, which are crosslinked along the heparin backbone, are more tightly crosslinked than the 10% Hep MPs, which crosslink only at the ends of each PEG-4Ac arm. Therefore, one rationale for the difference in BMP-2 release is the less tightly crosslinked network of 10% Hep MPs in comparison to 100% Hep MPs, allowing for greater diffusion and more abundant release of BMP-2. Prior studies with heparin-containing hydrogels resulted in a large range of release, between 20–80% of loaded BMP-215,43,44. In studies more similar to our system, heparin-containing MPs released 40–60% of loaded BMP-230,31, which is consistent with our findings from 10% Hep MPs. In these cases, however, heparin was either covalently attached to hyaluronic acid MPs or coated onto the alginate MP surface, rather than crosslinked into PEG-based MPs as in our system.
To further tune BMP-2 release from MPs we varied the degradation of MPs between ~10–14 days. However, regardless of the time course of MP degradation, similar release kinetics were observed from all MPs (Figure 4). These findings suggest that BMP-2 release was governed by diffusion rather than exclusively on the degradation of the MPs and led us to simultaneously alter sulfation pattern within the degradable MPs. Specifically, we hypothesized that with decreased heparin sulfation and concomitant reduction in electrostatic interactions within the MPs, more desulfated heparin MPs would release greater amounts of BMP-2 and thus provide a means to further tune BMP-2 release. Instead, as heparin sulfation levels within MPs decreased, the total BMP-2 release recorded also significantly decreased (Figure 4B–C). Additionally, C2C12 ALP activity indicated that MPs with more sulfated heparin derivatives, Hep and Hep−N, enhanced the bioactivity of BMP-2 over soluble BMP-2, whereas little ALP activity was stimulated by less sulfated heparin MPs. For the MPs that had not fully degraded by Day 10 (1 wt% Hep, Hep−N, and Hep−6O,N) it is possible that some BMP-2 was still entrapped beyond the timeframe of the 10 day release study, however, this does not explain the trends in ALP activity and BMP-2 release observed in the majority of the MP formulations. Interpreting our BMP-2 release and bioactivity results collectively, we hypothesized that BMP-2 was significantly more protected by more sulfated heparin MPs, resulting in greater detected levels of BMP-2 and enhanced BMP-2 bioactivity in these MP groups. This interpretation also applies to the differences between 10% and 1% heparin MPs, whereby the MPs with greater amounts of heparin (10 wt%) protected more BMP-2 than 1 wt% MPs with the same heparin species.
To test our hypothesis, we incubated all the heparin derivatives with BMP-2 in solution to investigate the effect of heparin on BMP-2 detection. After 24 hours at 4°C, the detected levels of BMP-2 remained between 90–100 ng in Hep and Hep−N samples but decreased significantly in Hep−6O,N, Hep-, and no heparin samples, suggesting that more sulfated heparin derivatives may protect BMP-2 from denaturation in this time frame (Figure S2A). Similar trends were also seen previously in our laboratory after heat treatment, where more BMP-2 protection was found with more sulfated soluble heparin derivatives, although these experiments were carried out with non-glycosylated BMP-2, unlike the glycosylated form used in these studies34. It is important to note, however, that even in the most sulfated heparin MP groups, some BMP-2 denaturation may have occurred over time, resulting in less than 100% detected release of loaded BMP-2.
Applying our bioactivity results to our MP studies, more desulfated heparin MPs were likely unable to protect the bound BMP-2, resulting in little release of detectable BMP-2 and minimal BMP-2 bioactivity after as little as 24 hours. In contrast, more sulfated heparin MPs, and particularly those with higher heparin content, were able to maintain BMP-2 bioactivity during release. However, in our MP experiments it is still unclear whether the source of BMP-2 protection lies in BMP-2 interactions with heparin within and on the surface of MPs or interactions with soluble heparin released from MPs. Therefore, quantifying the amount of heparin released from MPs over time may shed light on how BMP-2 is protected in this system.
Previous experiments have demonstrated that the FGF family of growth factors is protected by heparin45,46. However, compared with FGF, relatively few studies have investigated how sulfation pattern may contribute to heparin’s role in protecting BMP-2 bioactivity, and the results have been inconsistent34,47. One group has demonstrated that BMP-2 bioactivity was enhanced after incubating with a desulfated heparin derivative. This work used soluble 2O-desulfated heparin, which is distinct from any of our derivatives, and mesenchymal stem cells (MSCs) were used to assess BMP-2 bioactivity rather than C2C12 cells48. On the other hand, a separate set of studies have corroborated our findings where BMP-2 was bioactive after incubation with fully sulfated heparin and inactive with fully desulfated heparin, as determined via C2C12 ALP activity47. It should be noted, however, that only soluble heparin was used and the fully desulfated heparin was further altered to be N-acetylated or N-sulfated, unlike the Hep- used for our studies. In our studies, because multiple sulfate groups were removed simultaneously, we were unable to completely decouple the effects of sulfation level and sulfation pattern, although this may be an interesting avenue for future investigation. Thus, based on reported results as well as our findings in these experiments, heparin sulfation pattern/level may be an important consideration in fabrication of GAG-based delivery systems for BMP-2 in the future.
Conclusions
Hydrolytically-degradable, heparin-based MPs were fabricated containing heparin derivatives with varying levels of sulfation. It was demonstrated that MP degradation time in vitro can be adjusted by varying the heparin content (weight %) within MPs. Furthermore, our results indicate that most MP formulations load equivalent amounts of BMP-2, whereas more sulfated heparin MPs, Hep and Hep−N, are able to release significantly greater detectable levels of intact BMP-2 than more desulfated heparin MPs, Hep−6O,N and Hep-. Similarly, presentation of BMP-2 from more sulfated heparin MPs can enhance BMP-2 bioactivity compared to growth factor in solution, whereas heavily desulfated heparin MPs maintain little to no BMP-2 bioactivity. Therefore, we have identified 10 wt% Hep and Hep−N MPs as viable growth factor carriers capable of efficient loading and release of bioactive BMP-2, and demonstrated that heparin sulfation level may be an important consideration for any future heparin-based biomaterials approach for bioactive growth factor delivery.
Supplementary Material
Table 4.
Average size of microparticle formulations.
| Hep | Hep−N | Hep−6O,N | Hep- | |
|---|---|---|---|---|
| 10 wt% heparin MPs | 12.3 ± 7.7 | 13.2 ± 11.4 | 13.5 ± 9.7$ | 12.3 ± 7.0 |
| 1 wt% heparin MPs | 11.3 ± 9.3 | 11.8 ± 10.9$ | 12.8 ± 6.4 | 13.3 ± 6.8 |
Significantly different from each other; p ≤ 0.05; n > 150.
Table 5.
Degradation time (days) for all microparticle formulations.
| Hep | Hep−N | Hep−6O,N | Hep- | |
|---|---|---|---|---|
| 10 wt% heparin MPs | 7 | 10 | 10 | 10 |
| 1 wt% heparin MPs | 14 | 14 | 14 | 10 |
Acknowledgments
We would like to acknowledge Karthik Nathan for his work in developing the materials used in these studies. This research was funded by NSF DMR 1207045, NIH (R01 AR062006), and NSF Stem Cell Biomanufacturing IGERT (DGE 0965945).
Footnotes
Footnotes relating to the title and/or authors should appear here.
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Footnotes relating to the main text should appear here. These might include comments relevant to but not central to the matter under discussion, limited experimental and spectral data, and crystallographic data.
Notes and references
- 1.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) International orthopaedics. 2007;31(6):729–34. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaibhav B, et al. Bone morphogenic protein and its application in trauma cases: a current concept update. Injury. 2007;38(11):1227–35. doi: 10.1016/j.injury.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Kang SW, et al. Surface modification with fibrin/hyaluronic acid hydrogel on solid-free form-based scaffolds followed by BMP-2 loading to enhance bone regeneration. Bone. 2011;48(2):298–306. doi: 10.1016/j.bone.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 4.Tao L, et al. Synthesis and bioactivity of poly(HPMA)-lysozyme conjugates: the use of novel thiazolidine-2-thione coupling chemistry. Organic & biomolecular chemistry. 2009;7:3481–3485. doi: 10.1039/b907061c. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman JR, Daluski A, Einhorn TA. The role of growth factors in the repair of bone. Journal of Bone and Joint Surgery. 2002:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Sciadini MF, Johnson KD. Evaluation of Recombinant Human Bone Morphogenetic Protein-2 as a Bone-Graft Substitute in a Canine Segmental Defect Model. 2000:289–302. doi: 10.1002/jor.1100180218. [DOI] [PubMed] [Google Scholar]
- 7.Lim HJ, et al. Controlled release of BMP-2 from alginate nanohydrogels enhanced osteogenic differentiation of human bone marrow stromal cells. Macromolecular Research. 2010;18(8):787–792. [Google Scholar]
- 8.Bae IH, et al. Evaluation of a thiolated chitosan scaffold for local delivery of BMP-2 for osteogenic differentiation and ectopic bone formation. BioMed Research International. 2013 doi: 10.1155/2013/878930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel ZS, et al. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomaterialia. 2008;4:1126–1138. doi: 10.1016/j.actbio.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wehrhan F, et al. PEG matrix enables cell-mediated local BMP-2 gene delivery and increased bone formation in a porcine critical size defect model of craniofacial bone regeneration. Clinical Oral Implants Research. 2012;23:805–813. doi: 10.1111/j.1600-0501.2011.02223.x. [DOI] [PubMed] [Google Scholar]
- 11.Tabata Y, Yamamoto M, Ikada Y. Comparison of Release Profiles of Various Growth Factors from Biodegradable Carriers. 1998;530:13–18. [Google Scholar]
- 12.Jeon O, et al. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(l-lactic-co-glycolic acid) scaffold. Biomaterials. 2007;28:2763–2771. doi: 10.1016/j.biomaterials.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Kang SW, et al. Bone morphogenetic protein-2 enhances bone regeneration mediated by transplantation of osteogenically undifferentiated bone marrow-derived mesenchymal stem cells. Biotechnology Letters. 2008;30:1163–1168. doi: 10.1007/s10529-008-9675-8. [DOI] [PubMed] [Google Scholar]
- 14.Yang HS, et al. Heparin-Conjugated Fibrin as an Injectable System. Tissue Engineering Part A. 2010;16(4):1–10. doi: 10.1089/ten.TEA.2009.0390. [DOI] [PubMed] [Google Scholar]
- 15.Bhakta G, et al. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials. 2012;33(26):6113–6122. doi: 10.1016/j.biomaterials.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seto SP, Casas ME, Temenoff JS. Differentiation of mesenchymal stem cells in heparin-containing hydrogels via coculture with osteoblasts. Cell Tissue Res. 2012;347(3):589–601. doi: 10.1007/s00441-011-1265-8. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. Journal of biomaterials science Polymer edition. 2001;12:77–88. doi: 10.1163/156856201744461. (January 2015) [DOI] [PubMed] [Google Scholar]
- 18.Gandhi NS, Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chemical Biology and Drug Design. 2008;72:455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller T, et al. Molecular engineering of glycosaminoglycan chemistry for biomolecule delivery. Acta Biomater. 2014;10(4):1705–19. doi: 10.1016/j.actbio.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumann H, et al. Novel regio- and stereoselective O-6-desulfation of the glucosamine moiety of heparin with N-methylpyrrolidinone-water or N,N-dimethylformamide-water mixtures. Carbohydrate Research. 1998;308:381–388. doi: 10.1016/s0008-6215(98)00097-4. [DOI] [PubMed] [Google Scholar]
- 21.Habuchi H, Habuchi O, Kimata K. Sulfation pattern in glycosaminoglycan: Does it have a code? Glycoconjugate Journal. 2004;21:47–52. doi: 10.1023/B:GLYC.0000043747.87325.5e. [DOI] [PubMed] [Google Scholar]
- 22.Roy S, et al. Bioactivity screening of partially desulfated low-molecular-weight heparins: A structure/activity relationship study. Glycobiology. 2011;21(9):1194–1205. doi: 10.1093/glycob/cwr053. [DOI] [PubMed] [Google Scholar]
- 23.Shipp EL, Hsieh-Wilson LC. Profiling the Sulfation Specificities of Glycosaminoglycan Interactions with Growth Factors and Chemotactic Proteins Using Microarrays. Chemistry and Biology. 2007;14:195–208. doi: 10.1016/j.chembiol.2006.12.009. (February) [DOI] [PubMed] [Google Scholar]
- 24.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair and Regeneration. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 25.Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1997;11:51–9. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher JT. Heparan sulfate proteoglycans Heparan sulfate: growth control with a restricted sequence menu. 2001;108(3):357–361. doi: 10.1172/JCI13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. European journal of biochemistry/FEBS. 1996;237:295–302. doi: 10.1111/j.1432-1033.1996.0295n.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim SE, et al. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-β1: Implications for cartilage tissue engineering. Journal of Controlled Release. 2003;91:365–374. doi: 10.1016/s0168-3659(03)00274-8. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, et al. Preparation, structure and BMP-2 controlled release of heparin-conjugated hyaluronan microgels. Carbohydrate Polymers. 2011;86(2):806–811. [Google Scholar]
- 30.Xu X, et al. Heparin-decorated, hyaluronic acid-based hydrogel particles for the controlled release of bone morphogenetic protein 2. Acta Biomaterialia. 2011;7(8):3050–3059. doi: 10.1016/j.actbio.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbah SA, et al. Enhanced control of in vivo bone formation with surface functionalized alginate microbeads incorporating heparin and rhBMP-2. Tissue Engineering Part A. 2012;19:120815214523006–120815214523006. doi: 10.1089/ten.tea.2012.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hettiaratchi MH, et al. Heparin microparticles effects on presentation and bioactivity of bone morphogenetic protein-2. Biomaterials. 2014;35(25):7228–7238. doi: 10.1016/j.biomaterials.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bar-Ner M, et al. Inhibition of Heparanase-Mediated Degradation of Extracellular Matrix Heparan Sulfate by Non-anticoagulant Heparin Species. Blood. 1987;70(2):551–557. [PubMed] [Google Scholar]
- 34.Seto SP, Miller T, Temenoff JS. Effect of Selective Heparin Desulfation on Preservation of Bone Morphogenetic Protein - 2 Bioactivity after Thermal Stress. 2015 doi: 10.1021/bc500565x. [DOI] [PubMed] [Google Scholar]
- 35.Inoue Y, Nagasawa K. Selective N-desulfation of heparin with dimethyl sulfoxide containing water or methanol. Carbohydrate research. 1976;46:87–95. doi: 10.1016/s0008-6215(00)83533-8. [DOI] [PubMed] [Google Scholar]
- 36.Nagasawa K, Inoue Y, Kamata T. Solvolytic Desulfation of Glycosaminoglycuronan Sulfates With Dimethyl Sulfoxide Containing Water or Methanol. Carbohydrate Research. 1977;58:47–55. doi: 10.1016/s0008-6215(00)83402-3. [DOI] [PubMed] [Google Scholar]
- 37.Tae G, et al. Formation of a novel heparin-based hydrogel in the presence of heparin-binding biomolecules. Biomacromolecules. 2007;8:1979–1986. doi: 10.1021/bm0701189. [DOI] [PubMed] [Google Scholar]
- 38.McGonigle JS, et al. Heparin-regulated delivery of osteoprotegerin promotes vascularization of implanted hydrogels. Journal of biomaterials science Polymer edition. 2008;19:1021–1034. doi: 10.1163/156856208784909381. (February 2015) [DOI] [PubMed] [Google Scholar]
- 39.Hahn MS, et al. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials. 2006;27:2519–2524. doi: 10.1016/j.biomaterials.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 40.Katagiri T, et al. Morphogenetic Protein -2 Converts the Differentiation Pathway of C2C12 Myoblasts into the Osteoblast Lineage. Journal of Cell Biology. 1994;127(6):1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zalipsky S. Functionalized Poly(ethylene glycol) for Preparation of Biologically Relevant Conjugates. Bioconjugate Chemistry. 1995;6(2):150–165. doi: 10.1021/bc00032a002. [DOI] [PubMed] [Google Scholar]
- 42.Roman M, Winter WT. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules. 2004;5:1671–1677. doi: 10.1021/bm034519+. [DOI] [PubMed] [Google Scholar]
- 43.Chung YI, et al. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J Control Release. 2007;121(1–2):91–9. doi: 10.1016/j.jconrel.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 44.Jeon O, et al. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J Control Release. 2011;154(3):258–66. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gospodarowicz D, Cheng J. Heparin Protects Basic and Acidic FGF From Inactivation. Journal of Cellular Physiology. 1986;128:475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- 46.Kan M, et al. An Essential Heparin-Binding Domain in the Fibroblast Growth Factor Receptor Kinase. Science. 1993;259 doi: 10.1126/science.8456318. [DOI] [PubMed] [Google Scholar]
- 47.Takada T, et al. Sulfated Polysaccharides Enhance the Biological Activities of Bone Morphogenetic Proteins. Journal of Biological Chemistry. 2003;278(44):43229–43235. doi: 10.1074/jbc.M300937200. [DOI] [PubMed] [Google Scholar]
- 48.Ratanavaraporn J, Tabata Y. Enhanced osteogenic activity of bone morphogenetic protein-2 by 2-O-desulfated heparin. Acta Biomaterialia. 2012;8(1):173–182. doi: 10.1016/j.actbio.2011.09.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


