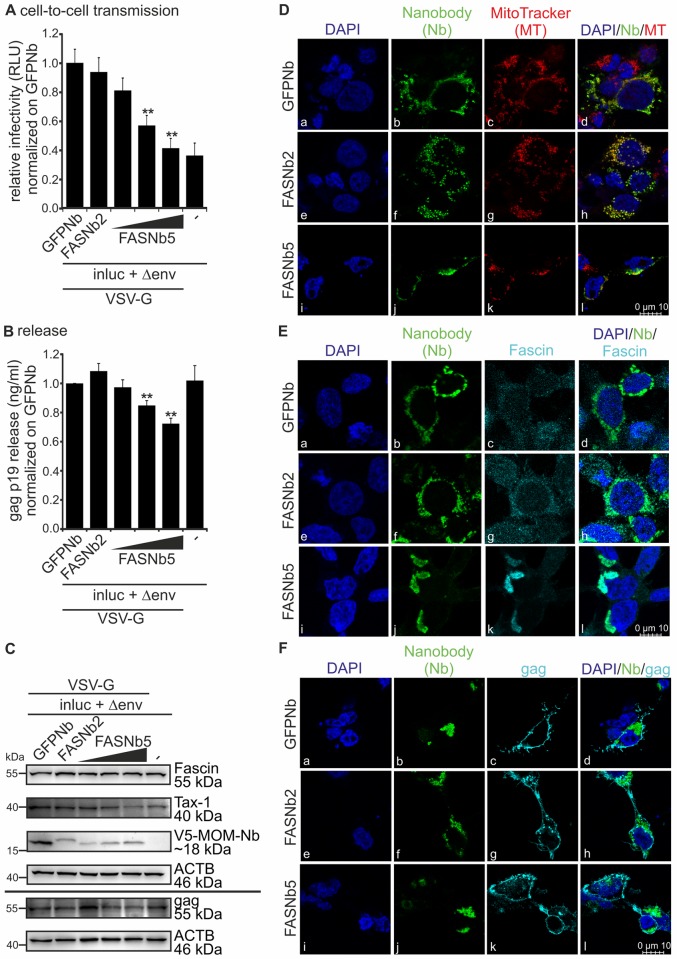
Fig 2. Inhibition of actin-bundling using Fascin-specific nanobodies displays a significant reduction of virus transmission.
(A-C) 293T cells were transfected with the reporter vector pCRU5HT1M-inluc (inluc) and the packaging plasmid pCMVHT1M-ΔEnv encoding all HTLV-1 proteins except env (Δenv) pseudotyped with VSV-G. Cells were co-transfected with V5-tagged expression plasmids encoding a mitochondrial outer membrane (MOM) sequence and nanobodies (Nb) targeting Fascin (MOMFASNb2 (FASNb2), 0.5μg; or MOMFASNb5 (FASNb5), 0.25; 0.5; 1μg), or a control nanobody (MOMGFPNb (GFPNb), 0.5μg). At 48h post transfection, luciferase assays, ELISA and western blot were performed as described in Fig 1A. The means of four independent experiments ± standard error (SE) are shown and were compared to the control (GFPNb) using Student’s t-test (**: p<0.01). (A) Luciferase activity (cell-to-cell transmission). (B) Detection of gag p19 release by ELISA. (C) Detection of Fascin, Tax-1, V5 (MOM-Nanobodies) and gag by western blot. β-actin (ACTB) served as control. (D-F) Confocal laser scanning microscopy of 293T cells co-transfected with pCMVHT1M-ΔEnv and with V5-tagged expression plasmids encoding a MOM-sequence and nanobodies FASNb2, FASNb5 or GFPNb (control). Stains of nanobodies (V5; green) and (D) mitochondria (red), (E) Fascin (turquoise), and (F) gag p19 (turquoise) and the merges of the respective stains are shown. DAPI (blue) served as control.