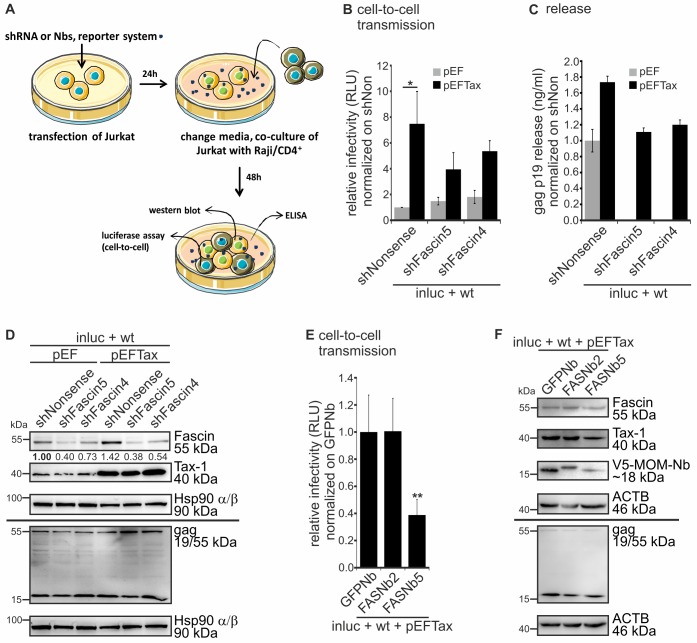
Fig 3. Repression of Tax-induced Fascin results in reduction of both virus release and cell-to-cell transmission.
(A) Scheme of experimental setup using single-cycle replication-dependent reporter vectors in Jurkat T-cells and Raji/CD4+ B-cells. (A-D) Jurkat T-cells were transfected with the reporter vector pCRU5HT1M-inluc (inluc) and the packaging plasmid pCMVHT1M encoding HTLV-1 with wildtype env (wt). Cells were co-transfected with pEFTax or pEF (mock) and one of two different shRNAs targeting Fascin (shFascin5, shFascin4) or the control (shNonsense). Luciferase assays, ELISA and western blot were performed as depicted in A). (B) Luciferase activity (cell-to-cell transmission). The means of four independent experiments ± standard error (SE) are shown and were compared to the respective mock (shNonsense+pEF or shNonsense+pEFTax) using Student’s t-tests (*: p<0.05). (C) Detection of gag p19 release by ELISA. A representative experiment is shown. (D) Detection of Fascin, Tax-1 and gag by western blot. Hsp90 α/β served as control. Numbers indicate densitometric analysis of Fascin detection normalized on Hsp90 α/β. (E-F) Jurkat T-cells were co-transfected with the reporter vector pCRU5HT1M-inluc (inluc), the packaging plasmid pCMVHT1M (wt), pEFTax, and one of three different V5-tagged expression plasmids encoding a MOM-sequence and nanobodies FASNb2, FASNb5 or GFPNb (control). Luciferase assays and western blot were performed as depicted in A). (E) Luciferase activity (cell-to-cell transmission). The means of six independent experiments ± standard error (SE) were normalized and compared to control samples (GFPNb) using Student’s t-tests (**: p<0.01). (F) Detection of Fascin, Tax-1, V5-tagged nanobodies and gag by western blot. β-actin (ACTB) served as control.