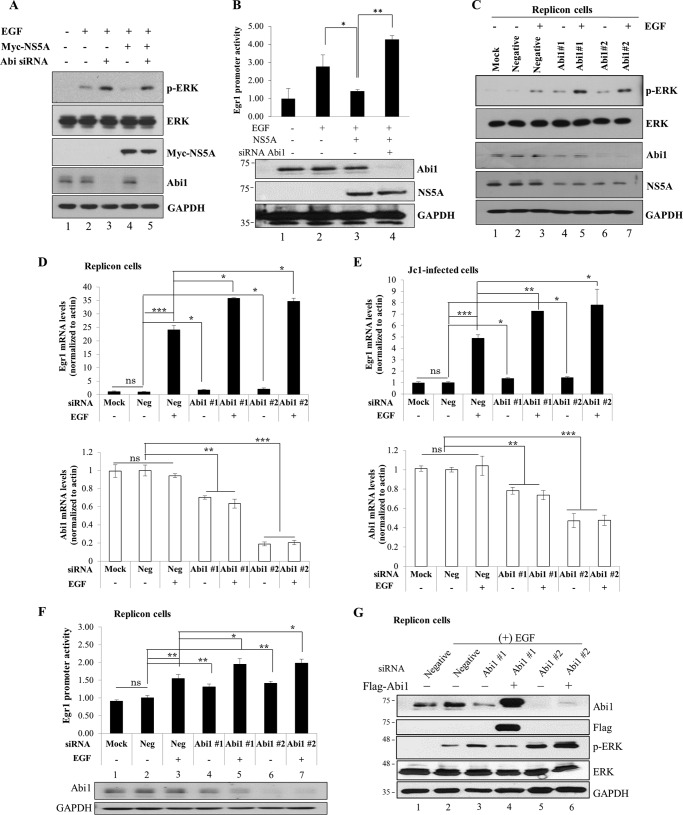
FIGURE 3.
NS5A down-regulates EGF signaling via modulation of Abi1. A, Huh7 cells were transfected with either negative control siRNA (−) or Abi1-specific siRNA (+). At 24 h after transfection, cells were transfected with 2 μg of Myc-tagged NS5A for 24 h. Cells were starved overnight and then treated with 100 ng/ml of EGF for 1 h. Total cell lysates were immunoblotted with the indicated antibodies. B, upper panel, Huh7 cells were transfected with either negative control siRNA or Abi1-specific siRNA. At 24 h after transfection, cells were further cotransfected with 2 μg of Myc-tagged NS5A and 1 ng of Egr1-luc promoter construct and 0.5 μg of pCH110. Cells were starved overnight and then treated with 100 ng/ml of EGF. Egr1 promoter activity was determined at 5 h after treatment. Experiments were carried out in triplicate. Error bars indicate the mean ± S.D. Lower panel, protein expressions in the same cell lysates were verified with the indicated antibodies. C, Huh7 cells harboring HCV subgenomic replicon were either mock-transfected or transfected with either negative control siRNA or 2 different Abi1-specific siRNA. At 48 h after transfection, cells were starved overnight and then either left untreated or treated with 100 ng/ml of EGF. Following 1 h incubation, cell lysates were immunoblotted with the indicated antibodies. D, Huh7 cells harboring HCV subgenomic replicon were transfected with siRNAs as described in C. At 48 h after transfection, cells were starved overnight and then either left untreated or treated with 100 ng/ml of EGF for 2 h. Both intracellular Abi1 and Egr1 mRNA levels were quantified by qRT-PCR. E, Huh7 cells were either mock-transfected or transfected with the indicated siRNAs. At 24 h after transfection, cells were infected with Jc1 for 4 h. At 48 post-infection, cells were starved for 24 h and then treated with EGF as described above. At 2 h after EGF treatment, both intracellular Abi1 and Egr1 mRNA levels were quantified by qRT-PCR. The asterisks indicate significant differences (ns, non-significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001) from the value for the negative control. Experiments were carried out in duplicate. Error bars indicate the mean ± S.D. F, upper panel, Huh7 cells harboring HCV subgenomic replicon were either mock-transfected or transfected with either negative control siRNA or 2 different Abi1-specific siRNA. At 24 h after transfection, cells were further cotransfected with Egr1-luc promoter and pCH110 plasmids for 24 h. Cells were starved overnight and then treated with 100 ng/ml of EGF. Egr1 promoter activity was determined at 5 h after treatment. Lower panel, protein expressions in the same cell lysates were verified with the indicated antibodies. Experiments were carried out in duplicate. Error bars indicate the mean ± S.D. G, Huh7 cells harboring HCV subgenomic replicon were transfected with the indicated siRNAs. At 24 h after transfection, cells were further transfected with either empty vector (−) or FLAG-tagged Abi1 plasmid. Cells were starved overnight and then treated with 100 ng/ml of EGF. Total cell lysates harvested at 48 h after plasmid transfection were immunoblotted with the indicated antibodies. Abi1 siRNA #1 targets to the 3′ UTR of Abi1, whereas Abi1 siRNA #2 binds to the sequence inside of coding region of Abi1. Experiments were carried out in duplicate.