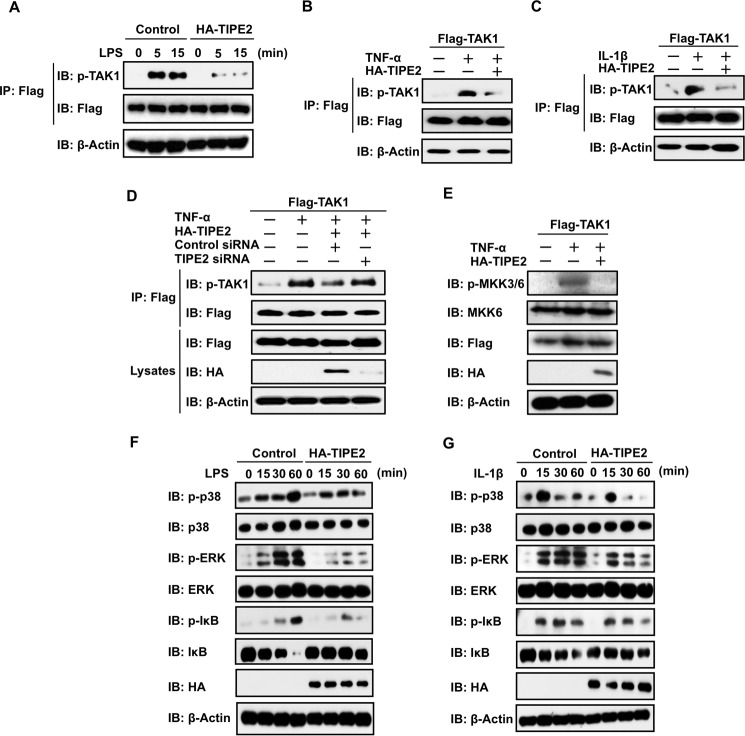
FIGURE 3.
TIPE2 inhibits phosphorylation of TAK1 and its downstream molecules in ligand-stimulated cells. A, TAK1-stable RAW246.7 cells were transfected with or without HA-TIPE2. The cells were incubated for 24 h and then incubated for an additional 24 h in serum-free culture conditions. Next, the cells were treated with or without LPS at 100 ng/ml. At the indicated times, the cells were extracted, and phosphorylation of TAK1 was detected by Western blotting assay using an anti-pTAK1 antibody. B and C, HEK293T cells were transfected with FLAG-TAK1 alone or FLAG-TAK1 with or without HA-TIPE2. After 24 h, the cells were incubated for an additional 24 h under serum-free culture conditions. The cells were then treated with or without TNF-α at 10 ng/ml (B) or IL-1β at 10 ng/ml (C) and extracted 5 min after the treatment. Then phosphorylation of TAK1 was detected by Western blotting assay using an anti-pTAK1 antibody. D, HEK293T cells were transiently transfected with FLAG-TAK1 alone or FLAG-TAK1 with or without HA-TIPE2, TIPE2 siRNA, and negative control siRNA. After 24 h, the cells were incubated for an additional 24 h in serum-free culture conditions and then treated for 5 min with or without TNF-α at 10 ng/ml. Then cells were extracted, and phosphorylation of TAK1 was detected by Western blotting assay using an anti-pTAK1 antibody. The expression of FLAG-TAK1 and HA-TIPE2 in whole cell lysates was detected by Western blotting assay. E, HEK293T cells were transfected with FLAG-TAK1 alone or FLAG-TAK1 with or without HA-TIPE2 vectors. After 24 h, the cells were incubated for an additional 24 h in serum-free culture conditions and then treated for 15 min with or without TNF-α at 10 ng/ml. TAK1 activity was monitored by detecting phosphorylation of MKK3/6 by Western blotting assay using an anti-pMKK3/6 antibody. The expression of FLAG-TAK1 and HA-TIPE2 in whole cell lysates was detected by Western blotting assay. F and G, TAK1-stable RAW246.7 cells were transfected with or without HA-TIPE2 vectors. After 24 h, the cells were incubated for an additional 24 h in serum-free culture conditions and then treated for the indicated times with or without LPS at 100 ng/ml (F) or IL-1-β at 10 ng/ml (G). Next, the cells were extracted at the indicated times, and phosphorylation of p38, ERK, and IκB was detected by Western blotting assay using anti-pp38, anti-pERK, and anti-pIκB antibodies. The expression of p38, ERK, and IκB in whole cell lysates was determined by Western blotting assay. IB, immunoblot; IP, immunoprecipitation.