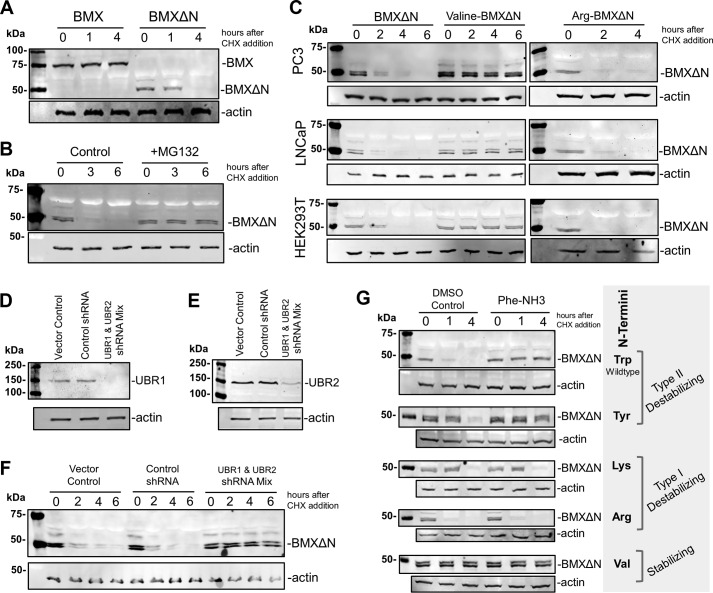
FIGURE 2.
BMXΔN is degraded by the N-end rule pathway. A, the stability of full-length BMX and BMXΔN was determined in transfected PC3 cells by treating the cells with 100 μg/ml CHX, to block protein synthesis, and then the cell cell lysates were analyzed by WB analysis at the indicated times. An anti-FLAG antibody was used to detect BMX, and an anti-actin antibody was used as a loading control. B, stability of BMXΔN was investigated in PC3 cells in the presence and absence of MG132 (10 μm) and analyzed as in A. C, wild type BMXΔN with N-terminal tryptophan (type II destabilizing N termini) and N-terminal mutants of arginine (type I destabilizing N termini) and valine (stabilizing N termini) were transfected into the cell lines indicated, and BMXΔN stability was determined as described in A. D, verification of shRNA knockdown of UBR1 in F by WB analysis with an anti-UBR1 antibody. E, verification of shRNA knockdown of UBR2 in F by WB analysis with an anti-UBR2 antibody. F, stability of BMXΔN was visualized in PC3 cells that also expressed shRNAs targeting UBR1 and UBR2, control shRNAs, or a pcDNA 3.1 vector control. G, stability of wild type BMXΔN and the listed N-terminal mutants in the presence and absence of 200 μm Phe-NH3 (preincubated for 4 h before the addition of CHX), an inhibitor reported to block the degradation of type II N termini while not affecting the degradation of proteins with type I N termini.