FIGURE 4.
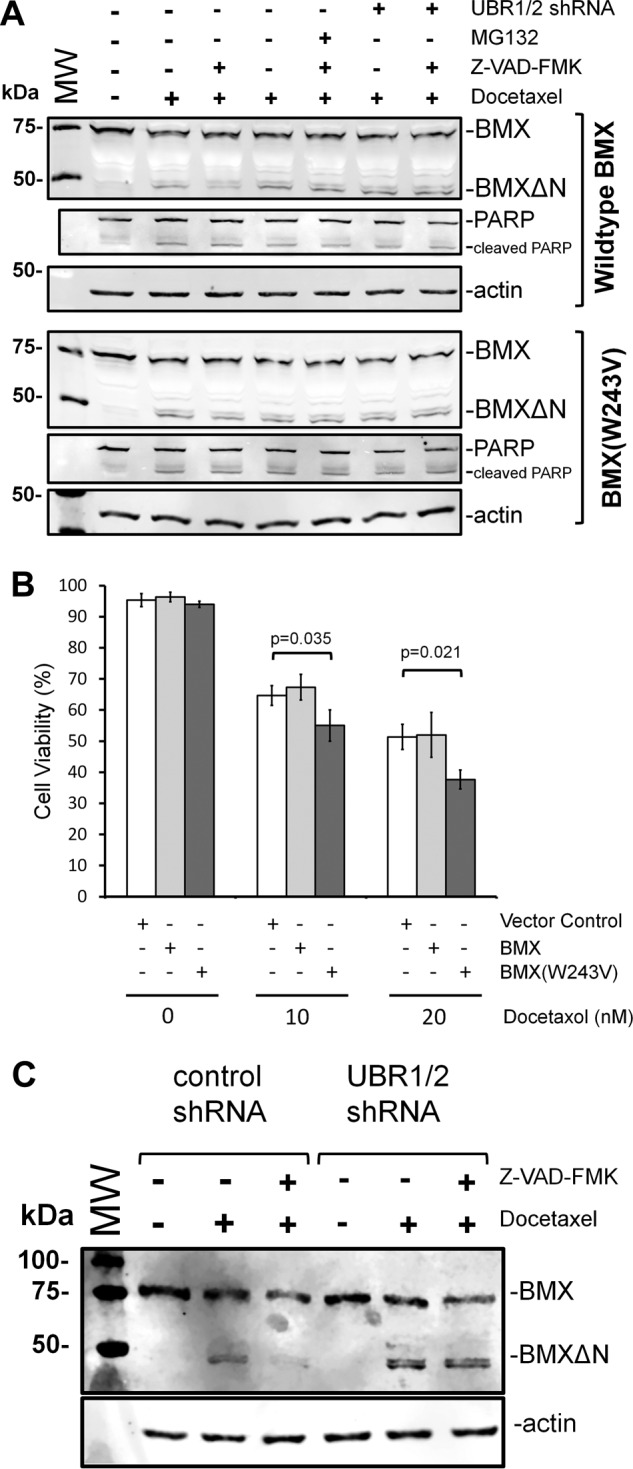
Cleavage of full-length recombinant or endogenous BMX and C-terminal fragment degradation. A, PC3 cells that stably express either shRNAs targeting UBR1 and UBR2 or shRNA controls were transfected to express full-length BMX (top) or the W243V mutant BMX. 24 h after transfection, the cells were either untreated or treated with 10 nm docetaxel for 48 h. The indicated samples were also treated with MG132 or Z-VAD-fmk. The pan-caspase inhibitor Z-VAD-fmk was added, for 2 h, to the indicated samples to prevent ongoing formation of BMXΔN by continued caspase activity. Western blotting analysis of cell lysates from the resulting experiments was performed to detect BMX and BMXΔN amounts, PARP, or an actin loading control. When Z-VAD-fmk was added to prevent the ongoing formation of BMXΔN, the disappearance of the C-terminal fragment is inhibited by knockdown of UBR1/2 with shRNAs or the W243V mutation, which results in a BMXΔN fragment with a stabilizing valine N terminus. B, PC3 cells expressing either recombinant full-length BMX or the W243V mutant were treated with the indicated amounts of docetaxel for 48 h and then analyzed by trypan blue staining. The data represent the average and S.D. (error bars) from three independent experiments, and p values were determined from paired two-tailed t tests. C, PC3 cells that stably express either shRNAs targeting UBR1 and UBR2 or shRNA controls were either untreated or treated with 10 nm docetaxel for 48 h to induce apoptosis. The pan-caspase inhibitor Z-VAD-fmk was added, for 3 h, to the indicated samples after docetaxel to prevent ongoing formation of BMXΔN by continued caspase activity. Western blotting analysis of cell lysates from the resulting experiments was performed to detect endogenous BMX and BMXΔN resulting from caspase cleavage.
