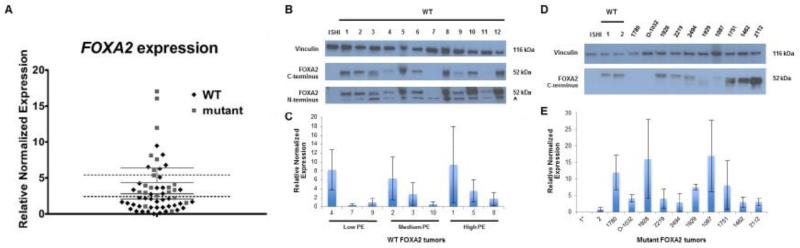
Fig. 2. Variable FOXA2 expression in EECs.
(A) mRNA levels in primary cancers, normalized to HepG2, shows >15 fold variability in FOXA2 transcripts. Expression was significantly higher in tumors with FOXA2 mutations (N=21, three missense, one in-frame deletion, and 17 stop or frameshift mutations) than in wild-type tumors (N=42), with mean expression levels 8.27 and 3.07, respectively (p ≤ 0.05). Mean relative mRNA expression level for each tumor was based on 3 different independent q-RT PCR experiments performed in quadruplicate. (B) Western blot analysis of FOXA2 using a C-terminal antibody (Abcam EPR4466). Protein levels varied from undetectable in a single tumor (lane 7) to high level expression, comparable to the HepG2 positive control. The Ishikawa cell line (negative control) does not express FOXA2. N-terminal antibody (Millipore 07-633) confirmed variability in protein levels, but because of a nonspecific band at ~36 kDa (arrowhead) it was not possible to reliably test for truncated protein products in mutant samples. (C) mRNA and protein levels are not correlated in FOXA2 wild-type tumors. (D) Western blot analysis of FOXA2 mutant tumor samples shows similar variability in expression of FOXA2 using a C-terminal antibody. Tumors 1780 and 0-1032 each have two mutations, and have no detectable FOXA2. The wild-type 52kDa protein is seen with the C-terminal antibody (stop and frameshift proteins not detectable). Tumors 1929 and 1087 are exceptions in that they have a single mutation (truncating), but have no or very little wild-type FOXA2. Wild-type endometrial tumors and HepG2 served as positive controls, and the Ishikawa cell line served as a negative control. Vinculin loading control. (E) No correlation between type of mutations, transcript levels, and protein expression for tumors with FOXA2 mutations. WT, wild-type; ISHI, Ishikawa cell line; PE, protein expression.