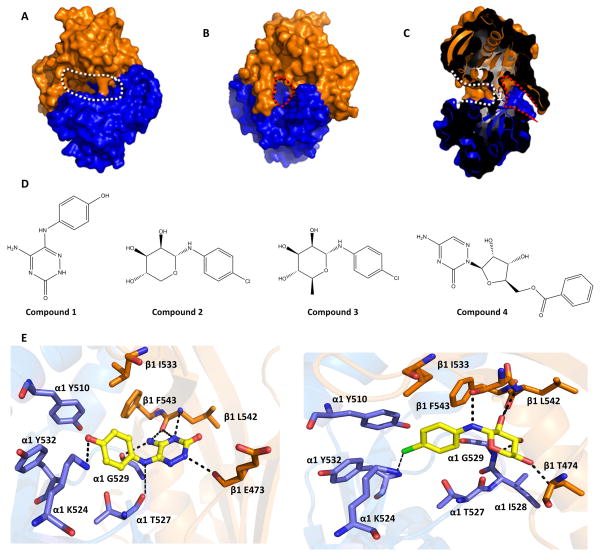
Fig 1. In silico screening targeting the “backside pocket” of sGC catalytic domain.
Molecular surface of the sGC catalytic domain showing the active site and backside pocket: A, View of the active site (outlined by white dotted line). The α1 catalytic domain is shown in blue, the β1 catalytic domain is in orange. B, Opposite face of the sGC catalytic domain showing the backside pocket (outlined by red dotted line). View in panel B is a 180° vertical rotation compared to A. C, Slabbed view of the catalytic domain dimer showing both the active site and the backside pocket. The view is obtained by a roughly 90° vertical rotation with respect to B. The active site and backside pocket are separated by a short segment of amino acids that includes residue α1 T527 (labeled as 1). D, Chemical structure of compounds 1–4. E, Predicted interactions of compound 1(left) and compound 2 (right) docking in the catalytic domain: hydrogen bonds are depicted as dashed lines. The terminal primary amine group of α K524 was not present in the structure of the catalytic domain (3UVJ), but was modelled in this figure as it can potentially form an interaction with chloride atom of compound 2. Similarly, the side chain of E473 was not included in the crystal structure but added here for illustrative purposes (this side chain is not anticipated to interact with either of the compounds). All the backside pocket residues are conserved in bovine and human sGC.