Fig. 1.
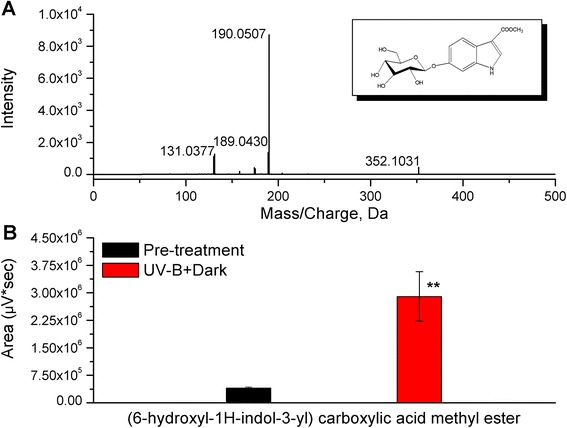
Identification of (6-hydroxyl-1H-indol-3-yl) carboxylic acid methyl ester in Clematis terniflora DC. leaves. Methanol extracts were obtained from C. terniflora leaves pre- and post-treatment with high level of UV-B irradiation for 5 h followed by an incubation for 36 h in the dark (UV-B + Dark), and then analyzed using HPLC-TOF-MS/MS. a HPLC-TOF-MS/MS spectrum of (6-hydroxyl-1H-indol-3-yl) carboxylic acid methyl ester (negative mode) post-treatment. b Statistics of peak area of (6-hydroxyl-1H-indol-3-yl) carboxylic acid methyl ester pre- and post-treatment. Data are shown as the mean ± SD from three independent biological replicates. Asterisks indicate significant differences as measured by Student’s t-test (**P < 0.01)
