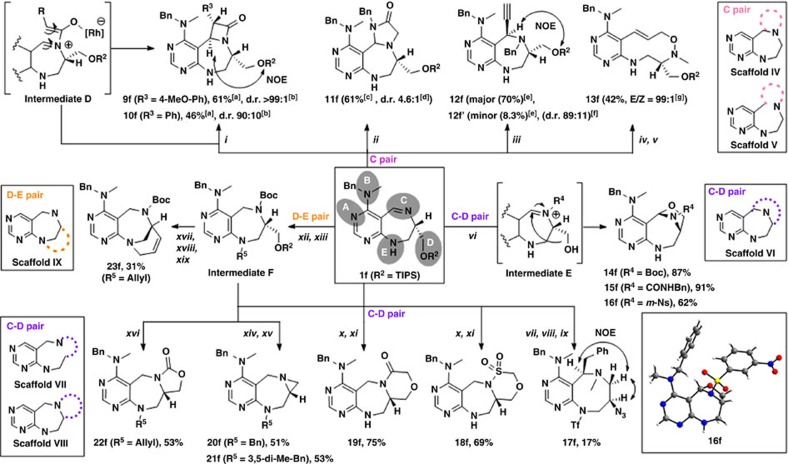
Figure 3. C, C–D and D–E paring pathways for synthesis of scaffolds IV–VII.
Reagents and conditions: (i) Rh(PPh3)3Cl (10 mol%), 4-picoline N-oxide, 4-ethynylanisole or phenylacetylene, ACN, μ-wave, 90 °C, 35 min; (ii) N-benzyl-2-chloroacetamide, NaBr, DMF, μ-wave, 110 °C, 30 min, then DBU, DMF; (iii) BnBr, ACN, 80 °C, then ethynylmagnesium bromide, THF, −78 °C→r.t.; (iv) MeI, ACN, 40 °C, then vinylmagnesium bromide, THF, −78 °C→r.t.; (v) m-CPBA, DCM, then 3-chlorobenzoic acid; (vi) HF/pyridine/THF, then electrophiles (Boc2O, benzyl isocyanate or m-NsCl), DCM; (vii) MeI, ACN, 40 °C, then benzylmagnesium bromide, THF, −78 °C→r.t.; (viii) TBAF, THF; (ix) Tf2O, toluene, −40 °C, then NaN3, −78 °C→r.t.; (x) NaBH4, MeOH, 0 °C →r.t., then chloromethane sulfonyl chloride or chloroacetic anhydride, TEA, DCM, 0 °C→r.t.; (xi) TBAF, THF, then Cs2CO3, DMF, 90 °C; (xii) NaBH4, MeOH, 0 °C→r.t., then Boc2O, TEA, DCM, 0 °C→r.t.; (xiii) BnBr, 3,5-dimethylbenzyl bromide or allyl bromide, NaH, DMF, 0 °C→r.t.; (xiv) TBAF, THF, then TFA, DCM; (xv) PS-PPh3, DEAD, THF; (xvi) TBAF, THF, then NaH, THF, 0 °C→r.t.; (xvii) TBAF, THF, then Dess–Martin periodinane, DCM; (xviii) Ph3P+CH3Br−, MeLi, THF, 0 °C→r.t.; (xix) Grubbs' second-generation catalyst (20 mol%), toluene, reflux. DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; m-CPBA, 3-chloroperbenzoic acid; m-NsCl, 3-nitrobenzenesulfonyl chloride; TBAF, tetra-n-butylammonium fluoride; Tf2O, trifluoromethanesulfonic anhydride; PS-PPh3, polystyrene triphenylphosphine; and DEAD, diethyl azodicarboxylate. [a]Yield of isolated major diastereomer. [b]Determined by LC-MS analysis of crude reaction mixture. [c]Yield of the mixture of diastereomers. [d]Determined by 1H NMR spectroscopy. [e]Yield of isolated diastereomer. [f]Determined from purified yield of each diastereomer. [g]Determined by LC-MS analysis of crude reaction mixture after treatment of 3-chlorobenzoic acid. 1H NMR, proton nuclear magnetic resonance; LC-MS, liquid chromatography-mass spectrometry.