Figure 8.
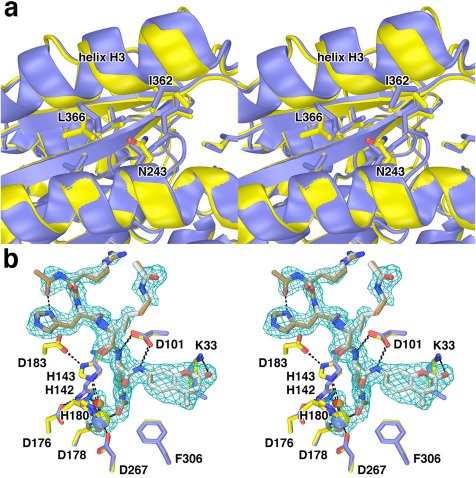
I243N HDAC8. (a) Comparison of the I243N HDAC8‐SAHA complex (yellow, C = yellow, N = blue, O = red) with the wild‐type HDAC8‐SAHA complex (blue; PDB 1T69). Selected residues are indicated. Helix H3 shifts by 0.3–1.6 Å as a result of the mutation. (b) Comparison of the I243N/Y306F HDAC8‐substrate complex (C = yellow (protein) or tan (substrate), N = blue, O = red, Zn2+ = yellow sphere) and the Y306F HDAC8‐substrate complex (C = blue (protein) or gray (substrate), N = blue, O = red, Zn2+ = blue sphere) (PDB 2V5W). Water molecules are indicated as red or orange spheres, respectively. Metal coordination and hydrogen bond interactions are shown as solid black and dashed black lines, respectively. The simulated annealing omit map is contoured at 3.0σ and shows a nearly fully ordered tetrapeptide substrate bound in the active site of I243N/Y306F HDAC8. Reprinted with permission from ref. 26. Copyright 2014, American Chemical Society.
