Abstract
The objective of this study was to four-dimensional (4D) print novel biomimetic gradient tissue scaffolds with highly biocompatible naturally derived smart polymers. The term “4D printing” refers to the inherent smart shape transformation of fabricated constructs when implanted minimally invasively for seamless and dynamic integration. For this purpose, a series of novel shape memory polymers with excellent biocompatibility and tunable shape changing effects were synthesized and cured in the presence of three-dimensional printed sacrificial molds, which were subsequently dissolved to create controllable and graded porosity within the scaffold. Surface morphology, thermal, mechanical, and biocompatible properties as well as shape memory effects of the synthesized smart polymers and resultant porous scaffolds were characterized. Fourier transform infrared spectroscopy and gel content analysis confirmed the formation of chemical crosslinking by reacting polycaprolactone triol and castor oil with multi-isocyanate groups. Differential scanning calorimetry revealed an adjustable glass transition temperature in a range from −8°C to 35°C. Uniaxial compression testing indicated that the obtained polymers, possessing a highly crosslinked interpenetrating polymeric networks, have similar compressive modulus to polycaprolactone. Shape memory tests revealed that the smart polymers display finely tunable recovery speed and exhibit greater than 92% shape fixing at −18°C or 0°C and full shape recovery at physiological temperature. Scanning electron microscopy analysis of fabricated scaffolds revealed a graded microporous structure, which mimics the nonuniform distribution of porosity found within natural tissues. With polycaprolactone serving as a control, human bone marrow-derived mesenchymal stem cell adhesion, proliferation, and differentiation greatly increased on our novel smart polymers. The current work will significantly advance the future design and development of novel and functional biomedical scaffolds with advanced 4D printing technology and highly biocompatible smart biomaterials.
Keywords: : 4D printing, smart polymer, shape memory, polycaprolactone, mesenchymal stem cell
Introduction
Tissue-engineered scaffolds are commonly defined as three-dimensional (3D) porous structures composed of biocompatible materials that perform multiple functions such as the promotion of cell adhesion and proliferation as well as directed tissue repair and regeneration.1 To achieve an ideal tissue-engineered scaffold, material selection and scaffold fabrication technique are extremely important.
Various materials with different physicochemical properties have been investigated for use in biomedical scaffolds, including metals, ceramics, polymers, and composites.2–8 Among these materials, shape memory polymers have attracted particular interest due to the potential for facile and minimally invasive surgical delivery with in situ shape activation for considerable reduction of trauma and significant improvement of patient comfort.9,10 In addition, seamless integration between the scaffold and defect would be better facilitated and addressed through the inherent shape memory effect.9
Several methods for 3D porous scaffold fabrication have been explored, including solid freeform fabrication, electrospinning, thermally induced phase separation, solvent casting/particle leaching, microsphere sintering, and scaffold coating.11–16 Among these fabrication techniques, 3D printing has garnered greater attention due to its excellent control of scaffold shape and interconnected porosity.17–19 Briefly, scaffolds are printed layer-by-layer based on predesigned computer-aided-design (CAD) files.19 Axial movement of the printing head is precisely controlled allowing for accurate and precise pore size, shape, and interconnectivity, which can be extended toward the fabrication of patient-specific defects.
Building on 3D printing technologies, four-dimensional (4D) printing is an emerging new concept that refers to the ability of 3D printed objects to change form and/or function after fabrication, thereby offering additional capabilities and/or performance-driven applications.20 For example, hydrophilic materials have been utilized to 4D fabricate self-evolving structures that perform geometric folding, curling, expansion, and various other programmed shape changes after submersion in water; active composite materials are prepared by printing glassy shape memory polymer fibers in an elastomeric matrix, and the 4D effect is realized by programmed action of the shape memory fibers.20,21
The 3D printing of shape memory biomedical scaffolds will have great potential for regenerative medicine in view of the combined advantages of 3D printing and the time-dependent shape memory effect. However, to the best of our knowledge, smart biomedical scaffolds fabricated by 4D printing with shape memory polymers have not been reported.
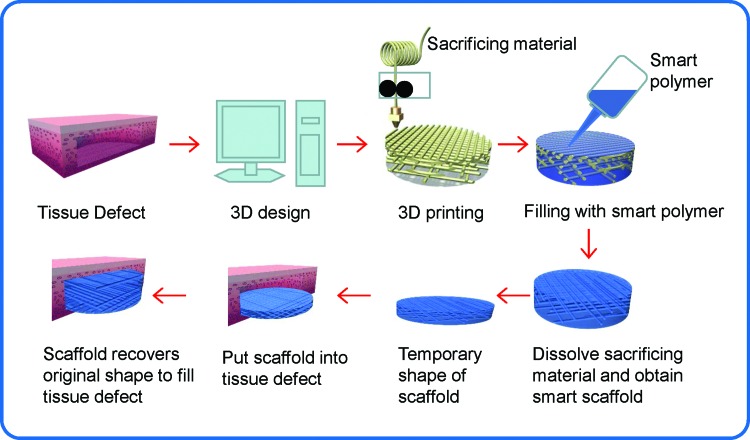
Therefore, in the current study, biomimetic hierarchical scaffolds were 4D fabricated from highly biocompatible smart polymers to serve as dynamic scaffolds for regenerative medicine as schematically shown in Figure 1. In comparison to common biomedical scaffolds that mostly have uniformly distributed porosity, 3D printing was utilized to achieve a gradient distribution of porosity from the periphery to the center of the construct to mimic natural tissues such as bone. The materials used in the study are smart biopolymers that were developed in our laboratory and exhibit excellent shape memory effects and shape recovery at physiological temperature. The biopolymers have also shown extraordinary biocompatibility with excellent attachment, proliferation, and differentiation of mesenchymal stem cells (MSCs). The results of this research have shed a light on the future design and development of novel and functional biomedical scaffolds with advanced 4D printing technology and highly biocompatible smart biopolymers.
FIG. 1.
The illustration of the process for preparing biomedical scaffold according to a specific traumatic defect. Color images available online at www.liebertpub.com/tec
Materials and Methods
Synthesis of smart polymer
Predetermined amounts of castor oil were mixed with polycaprolactone triol [number average molecular weight of 300 (Ptriol300) or 900 (Ptriol900)] and a crosslinker [either hexamethylene diisocyanate (HD) or poly(hexamethylene diisocyanate) (PH)] homogeneously in a glass beaker at room temperature and then heated to 60°C. The mixture was then poured into a polystyrene box and degassed to remove air bubbles. The plastic box was covered with a lid and put into a 60°C oven. The curing process was allowed to proceed for 48 h and the temperature was then increased to 70°C for an additional 24 h. The polymers were removed and allowed to sit at room temperature for a minimum of 24 h before analysis. Samples were coded according to the ratio of castor oil to Ptriol, Ptriol molecular weight, and the crosslinker type. For example, sample C80P300PH was composed of an 80:20 weight ratio of castor oil to Ptriol300 with PH serving as the crosslinker. In total, 22 samples were synthesized.
Porous scaffold fabrication
Interconnected porous scaffolds were designed in Rhinoceros 3D (McNeel North America, Seattle, Washington), prepared for 3D printing using the open source software package Slic3r, and 3D printed via a Solidoodle Workbench Apprentice 3D printer (Solidoodle, Brooklyn, New York). Poly(lactic acid) (PLA) molds were printed with the following parameters; filament diameter: 1.75 mm; extrusion multiplier: 1; nozzle diameter: 250 μm; extruder temperature: 175°C; bed temperature: 75°C; layer height: 0.3 mm; and solid infill.
To achieve a graded structure, the infill density was changed. Specifically, 25% was used for the first and second layer; 30% for the third and fourth layers; 35% for the fifth and sixth layers; and 40% for the seventh and eighth layers. After printing, the PLA structure was filled with the heated reaction mixture for synthesizing smart polymers. The same curing process was used as described previously. The graded porous scaffold was obtained after the PLA was removed by dissolving with dichloromethane and acetone sequentially.
Polymer and scaffold characterization
An FTIR spectrometer (Nicolet Series II Magna-IR System 750; Nicolet Instrument, Inc.) equipped with a horizontal germanium attenuated total reflectance accessory (ATR-FTIR) was used to evaluate all samples. The scan range used was 600 to 4000 cm−1 with a resolution of 4 cm−1.
Sol–gel analysis was performed according to a reported method with slight modification.22 Briefly, a 0.5 g sample was placed in 20 mL acetone and allowed to swell for a day at room temperature, and another 48 h at 50°C. The swollen gel was removed and dried at 60°C for 48 h. The gel content was determined as the weight of the dried sample divided by the total weight of the original sample.
Surface wettability of test specimens was measured using a contact angle analyzer (DSA4; Krüss). Approximately 3 μL of ultrapure H2O was deposited on the samples' surface and recorded. Static contact angle measurements were obtained from the first image of every recording. All experiments were conducted in ambient conditions and repeated five times per sample.
Surface morphology characterization of the smart polymer and porous structure of fabricated scaffolds was observed via a focused ion beam operating in scanning electron microscopy (SEM) mode (Zeiss NVision 40 FIB) under an accelerating voltage of 1–2 kV. The scaffold was cut with a scalpel to observe the internal interconnected pores and gold sputter coated before imaging.
The glass transition temperature (Tg) of synthesized polymers was measured with a multicell differential scanning calorimeter (MC DSC) from TA Instruments (New Castle, DE) at a programmed ramp rate of 1°C/min. The sample was first heated from 25°C to 150°C and held at 150°C for 1 min. Next, the sample was cooled from 150°C to −30°C and held at −30°C for 1 min. A second cycle was conducted: heating from −30°C to 150°C, holding 1 min and decreasing from 150°C to −30°C where results from this second cycle were used to determine the Tg.
Uniaxial compression tests were conducted using a uniaxial mechanical tester from MTS Systems Corporation (Eden Prairie, MN). Briefly, a flat 2-cm-diameter platen attached to a 100 N load cell was advanced on the sample (8-mm-diameter cylinder, 2 mm high) at a test speed of 10 mm/min and strain endpoint of 5 mm/mm. Data were taken using LabView (National Instruments Corporation, Austin, TX), and Young's modulus was determined by the linear elastic region.
Shape memory tests were conducted according to a reported method with slight modification.23 The polymer specimens were cut into rectangular strips measuring 75 × 10 × 2 mm. The edges of the strips were stained with black dye for increased optical contrast. The strips were folded 180° at 37°C into a “U” shape with a mold possessing an inner radius of 10 mm and kept at this temperature for 10 min. The samples were then cooled down immediately to a predetermined temperature (0°C or −18°C) and maintained at temperature for an additional 10 min. The mold was removed and the test strips were kept at temperature for an additional 10 min. The fixed angle of the specimen was determined and recorded as θfixed. The strips were then immersed in 37°C phosphate-buffered saline (PBS) immediately to recover the permanent shape. The time evolution of the specimen angle was determined by image processing and plotted vs time to quantitatively evaluate the transition speed. The final angle of the specimen was determined and recorded as θfinal. Shape fixity (Rf) and shape recovery (Rr) were calculated by the following equations:
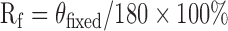 |
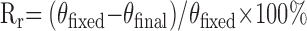 |
Human bone marrow MSC biocompatibility
Primary human bone marrow MSCs were obtained from healthy consenting donors at the Texas A&M Health Science Center, Institute for Regenerative Medicine. MSCs (passage no. 3–6) were cultured in complete media composed of alpha minimum essential medium (Gibco) supplemented with 16.5% fetal bovine serum (FBS) (Atlanta Biologicals), 1% (v/v) l-glutamine (Invitrogen), and 1% penicillin:streptomycin solution (Invitrogen) and cultured under standard cell culture conditions (37°C, a humidified, 5% CO2/95% air environment).
For MSC adhesion studies, the polymer test samples were cut into 8-mm-diameter specimens. MSCs were seeded at a cell density of 50,000 cells/specimen and cultured under standard cell culture conditions for 4 h. The specimens were then washed three times with PBS to remove nonadherent cells. Attached cells were lifted with trypsin–ethylenediaminetetraacetic acid, quantified with CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay, and analyzed spectrophotometrically using a Thermo Scientific Multiskan GO Spectrophotometer at 490 nm.
For proliferation studies, MSCs were seeded at a density of 10,000 cells/scaffold and cultured for 1, 3, and 5 days, respectively. Media were exchanged every other day and adhered cells were quantified as previously described. In addition, confocal microscopy was used to qualitatively examine MSC growth and spreading morphology. At each time point, samples were washed twice with PBS, fixed with 10% formalin, and permeabilized in 0.1% Triton X-100. After rinsing with PBS, cells were stained with Texas red fluorescent dye (to stain the cells' cytoskeleton) for 1 h and then DAPI blue fluorescent dye (to stain the cells' nuclei) for 15 min and imaged on a Zeiss LSM 710 confocal microscope.
For MSC osteogenic differentiation, MSCs were seeded at a density of 200,000 cells/cm2. Osteogenic differentiation media [DMEM supplemented with 10% FBS, 1% penicillin–streptomycin, 50 mg/mL l-ascorbate acid (Sigma), and 10 mM b-glycerophosphate (Sigma)] were used to culture the cells for 7, 14, and 21 days. At each predetermined time point, the samples were moved into a new well plate and rinsed with 50 mM Tris-buffered saline. MSCs were lysed using distilled water and three freeze–thaw cycles to disrupt the cell membrane and allow for intracellular and membrane-bound protein release.24
The biological activity of alkaline phosphatase (ALP) in the disrupted solution was measured with a QuantiChrom™ ALP Assay Kit (BioAssay Systems, Hayward, CA). The enzyme activity of ALP was set in relation to the total concentration of protein in solution, which was determined with a bicinchoninic acid assay (Micro BCA Protein Assay Kit; Thermo Scientific, Rockford, IL) to calculate the ALP-specific activity (mmol/mg/h).
Calcium deposition on different materials was measured using a Calcium Reagent Kit (Pointe Scientific, Inc.). Briefly, the material scaffold was immersed in a 0.6 N hydrogen chloride (HCl) solution at 37°C for 24 h. By reacting with o-cresolphthalein complex one, the calcium in the acidic supernatant was quantified. The absorbance at 570 nm was determined, and the calcium was calculated by standard curves of known calcium concentrations.
Statistical analysis
All data are expressed as mean ± standard deviation. The statistical significance was analyzed by a one-way analysis of variance test followed by using Tukey's pairwise comparison of the means. A significance level of p < 0.05 was used for all analyses.
Results
Synthesis and characterization of smart polymer
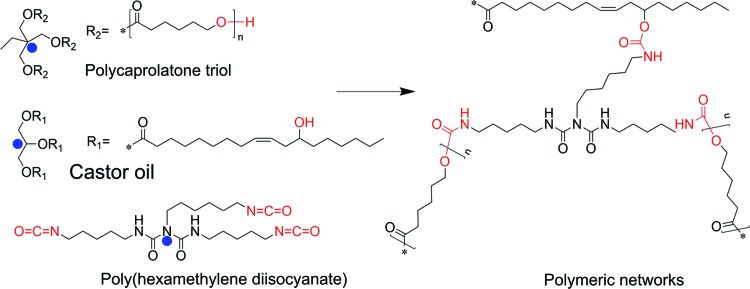
The reaction mechanism is schematically shown in Figure 2. The hydroxyl groups in Ptriol and castor oil additively react with isocyanate groups in HD or PH to form urethane bonds. Theoretically, there are two polymeric networks (Ptriol and castor oil-based networks, respectively) and three types of crosslinking points (the blue points in Fig. 2) in the resultant polymeric networks, expected to generate excellent shape recovery effects with widely tunable properties. By varying the ratio of components, a series of smart polymers have been prepared with all formulations presented in Table 1.
FIG. 2.
The reaction mechanism for synthesis of smart polymers. Color images available online at www.liebertpub.com/tec
Table 1.
The Constitutions of the Smart Polymers
| Sample code | castor oil/g | Ptriol300/g | Ptriol900/g | HD/g | PH/g | castor oil:Ptriola | SME |
|---|---|---|---|---|---|---|---|
| Castor100HD | 12.0676 | 2.9324 | 100:0 | ||||
| Castor100PH | 9.9338 | 5.0662 | 100:0 | ||||
| Ptriol900HD | 11.5186 | 3.4814 | 0:100 | ||||
| Ptriol900PH | 9.1781 | 5.8219 | 0:100 | ||||
| Ptriol300HD | 7.8669 | 7.1331 | 0:100 | ||||
| Ptriol300PH | 5.1670 | 9.8329 | 0:100 | ||||
| C80P300HD | 8.6912 | 2.1728 | 4.1359 | 80:20 | |||
| C80P300PH | 6.7092 | 1.6773 | 6.6136 | 80:20 | Yes | ||
| C80P900HD | 9.5253 | 2.3813 | 3.0933 | 80:20 | |||
| C80P900PH | 7.8181 | 1.9545 | 5.2274 | 80:20 | |||
| C60P300HD | 5.9662 | 3.9775 | 5.0563 | 60:40 | |||
| C60P300PH | 4.3537 | 2.9025 | 7.7438 | 60:40 | Yes | ||
| C60P900HD | 7.1034 | 4.7356 | 3.161 | 60:40 | |||
| C60P900PH | 5.7703 | 3.8469 | 5.3828 | 60:40 | |||
| C40P300HD | 3.6559 | 5.4838 | 5.8603 | 40:60 | Yes | ||
| C40P300PH | 2.5578 | 3.8366 | 8.6056 | 40:60 | Yes | ||
| C40P900HD | 4.6930 | 7.0395 | 3.2675 | 40:60 | |||
| C40P900PH | 3.7864 | 5.6797 | 5.5339 | 40:60 | |||
| C20P300HD | 1.6911 | 6.7644 | 6.5445 | 20:80 | |||
| C20P300PH | 1.1431 | 4.5725 | 9.2844 | 20:80 | Yes | ||
| C20P900HD | 2.3249 | 9.2994 | 3.3757 | 20:80 | |||
| C20P900PH | 1.8640 | 7.4562 | 5.6798 | 20:80 |
Weight ratio of castor oil to Ptriol.
SME, shape memory effect.
For all synthesized samples, the molar ratio of hydroxyl group to isocyanate group is kept constant at 1:1.05. The compatibility among the constituents plays a key role in preparing the polymeric networks. Most synthesized samples formed transparent films with Ptriol300HD and C20P300HD, the exceptions due to immiscibility of the reagents that yielded a highly viscous mixture with noticeable agglomerations. All other samples were screened for their shape memory effects. For this purpose, the samples were folded into a “U” shape at 37°C and their shape fixities determined at 0°C or −18°C. If the Rf is <90%, the sample is considered as having no shape fixity effect. As indicated in Table 1, five samples were found to possess a shape fixing effect and further investigated.
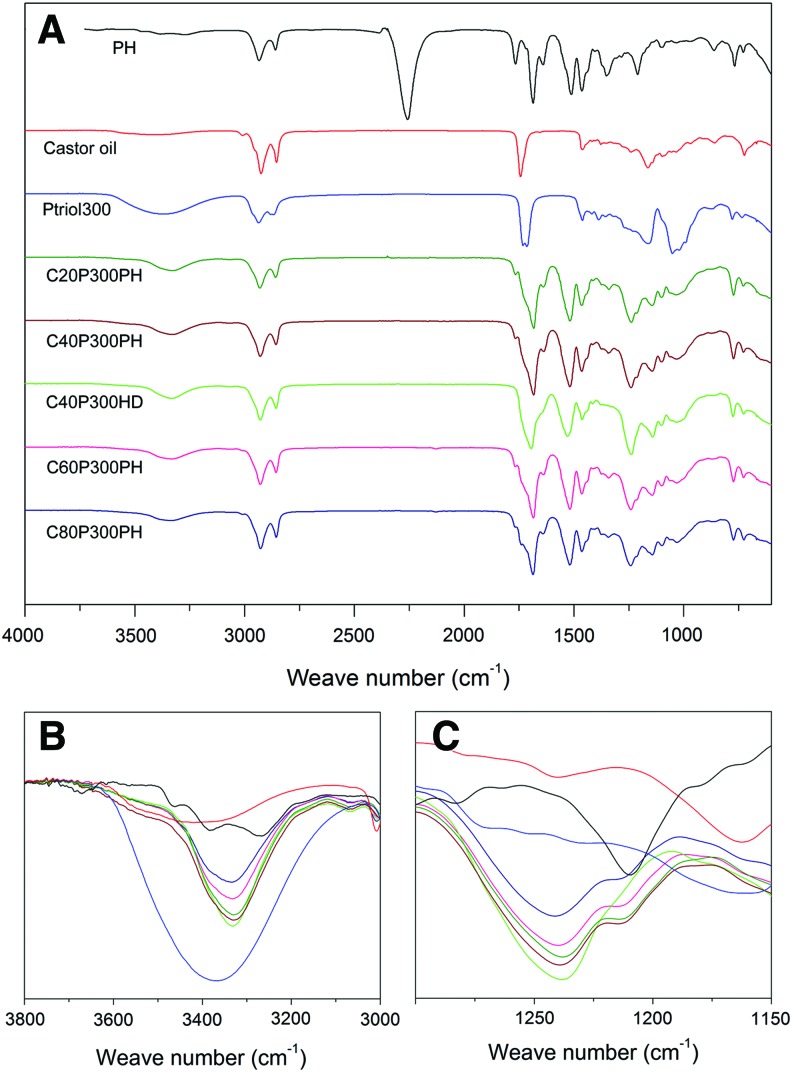
The formation of the urethane bonds in the five samples exhibiting shape memory effect was confirmed by FTIR analysis as shown in Figure 3. The signal at 2256 cm−1 in PH corresponds to the isocyanate group and is absent in the smart polymers thus implying complete consumption of isocyanates (Fig. 3A). The signal at 3368 cm−1 in Ptriol300 is attributed to the replacement of hydroxyl groups by the amide N–H stretching signal at 3329 cm−1 in smart polymers, as shown in Figure 3B, indicating the reaction of hydroxyl groups and formation of urethane bonds. The presence of a characteristic polyurethane peak in the amide I region 1650–1760 cm−1 corresponding to C = O stretching vibration is not clearly visible in the synthesized smart polymrs due to the signal overlaps with the neighboring C = O stretching vibration in PH. However, the formation of urethane bonds was further confirmed by the presence of a peak at 1238 cm−1 due to C–N stretching, where this peak is absent in castor oil, Ptriol300, and PH, as shown in Figure 3C.
FIG. 3.
FTIR spectra of five smart polymers when compared to PH, castor oil, and Ptriol300. (A) The FTIR spectra from 4000 to 600 cm−1; (B) The range from 3800 to 3000 cm−1; (C) The range from 1350 to 1150 cm−1. Color images available online at www.liebertpub.com/tec
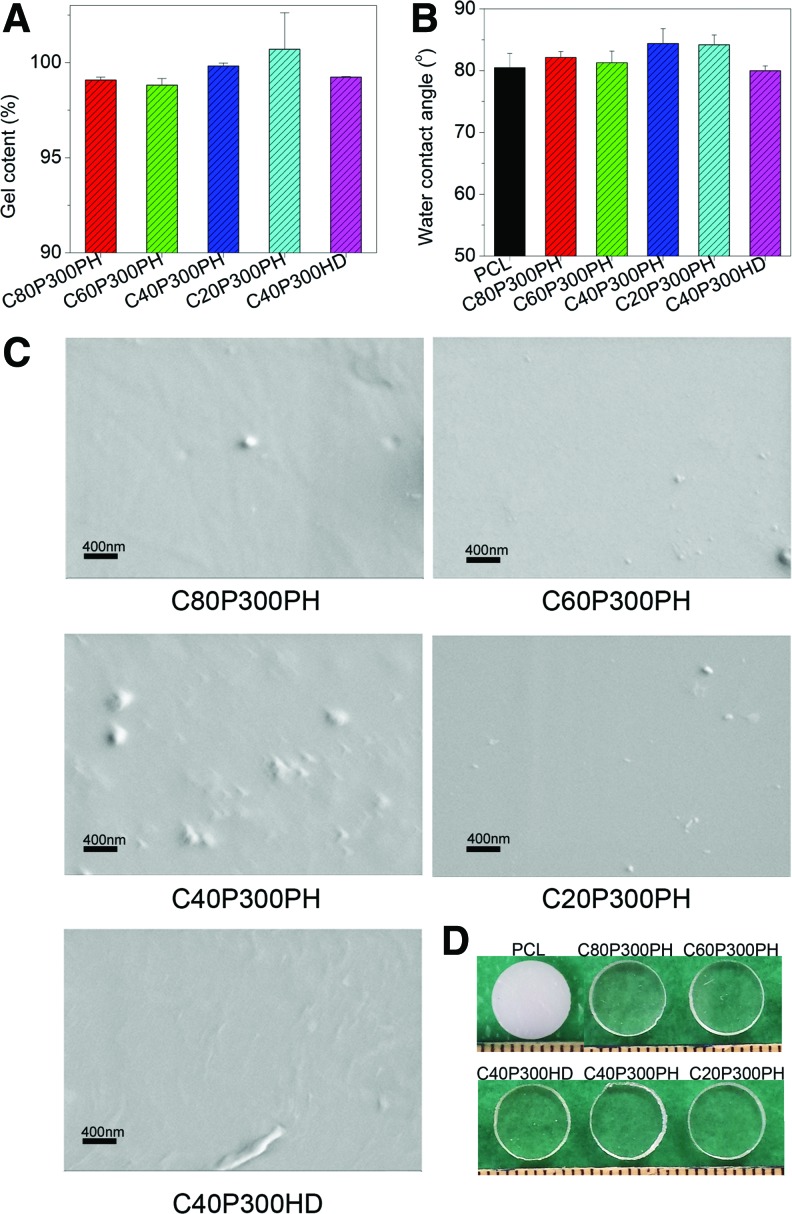
The gel content of synthesized smart polymers is shown in Figure 4A indicating that the polymers are fully crosslinked with gel contents greater than 95%. Contact angle analysis is shown in Figure 4B with no statistical difference observed between the smart polymers and polycaprolactone (PCL) control. Surface morphology was evaluated via SEM and is shown in Figure 4C. No noticeable surface topography changes were observed, suggesting that the smart polymers do not exhibit specific surface structures. Optical clarity can be seen in Figure 4D where all five smart polymers are transparent when compared to PCL.
FIG. 4.
Gel content (A), water contact angle (B), SEM (C), and photo images (D) the synthesized smart polymers. SEM, scanning electron microscopy. Color images available online at www.liebertpub.com/tec
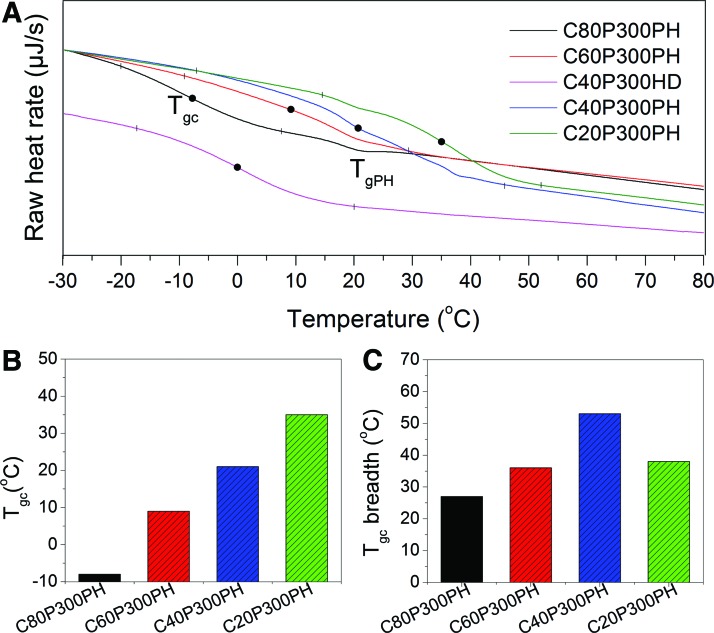
The Tgs of smart polymers was determined by DSC and the results are shown in Figure 5. A Tg of 21°C was observed in all the samples with PH as crosslinker, while no glass transition was observed at this temperature for sample C40P300HD. Therefore, this glass transition (TgPH) is assumed to be a function of the PH chains, where TgPH is not significantly affected by the sample composition. In contrast, the Tg from the incorporation of castor oil and Ptriol300 (Tgc) is proportional to castor oil content. As shown in Figure 5A and B, the Tgc shifts from −8°C to 35°C when the ratio of castor oil to Ptriol300 decreases from 80:20 to 20:80, which implies that these smart polymers are suitable for physiological temperature-triggered shape change. No other glass transition is clearly visible except TgPH and Tgc, implying that castor oil and Ptriol300 are highly miscible and well distributed within the samples. Tgc is clearly distinct from TgPH in sample C80P300PH, which indicates a phase separation between PH and other components. This separation is not distinguishable among all other samples as noted in sample C40P300PH where an overlap of Tgc and TgPH is observed. The Tgc breadth (the temperature range between Tgc onset and offset) is dependent on the castor oil content. As shown in Figure 5A and C, sample C40P300PH has the greatest Tgc breadth.
FIG. 5.
(A) DSC curves of the synthesized smart polymers; DSC, differential scanning calorimeter. (B) The effect of castor oil content on the Tgc. (C) The effect of castor oil content on the Tgc breadth. Color images available online at www.liebertpub.com/tec
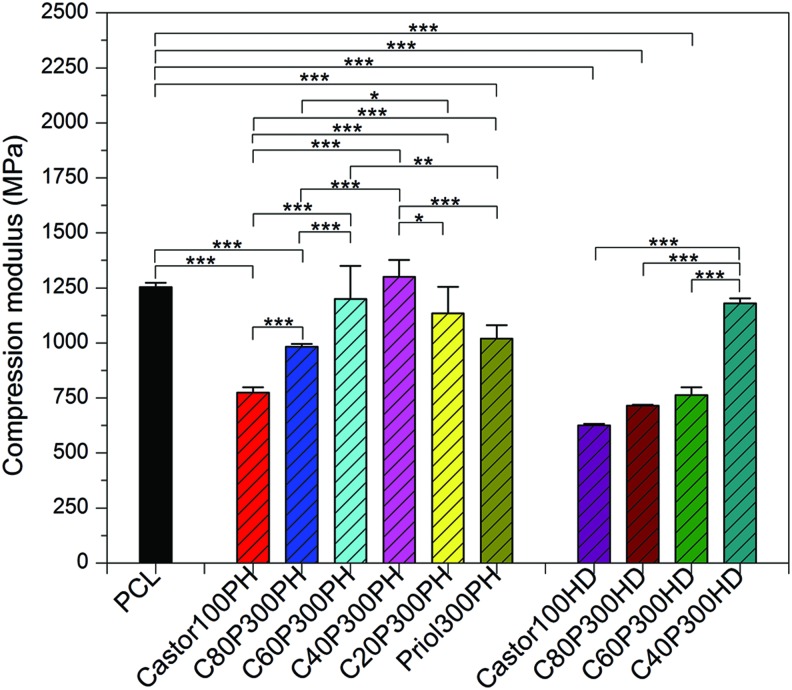
The mechanical behavior of the smart polymers was characterized via uniaxial compression testing and the results are shown in Figure 6. With PH as the crosslinker, sample C40P300PH showed the highest compression modulus. It was also noted that the compressive modulus increased when the weight ratio of castor oil to Ptriol300 decreased to a 40:60 mixture with samples C20P300PH and Ptriol300PH exhibiting a lower modulus than sample C40P300PH. With HD as the crosslinker, sample C40P300PH showed the highest compressive modulus.
FIG. 6.
Compression modulus of the synthesized smart polymers. Data are mean ± standard deviation, n = 5. *p < 0.05, **p < 0.01, and ***p < 0.001. Color images available online at www.liebertpub.com/tec
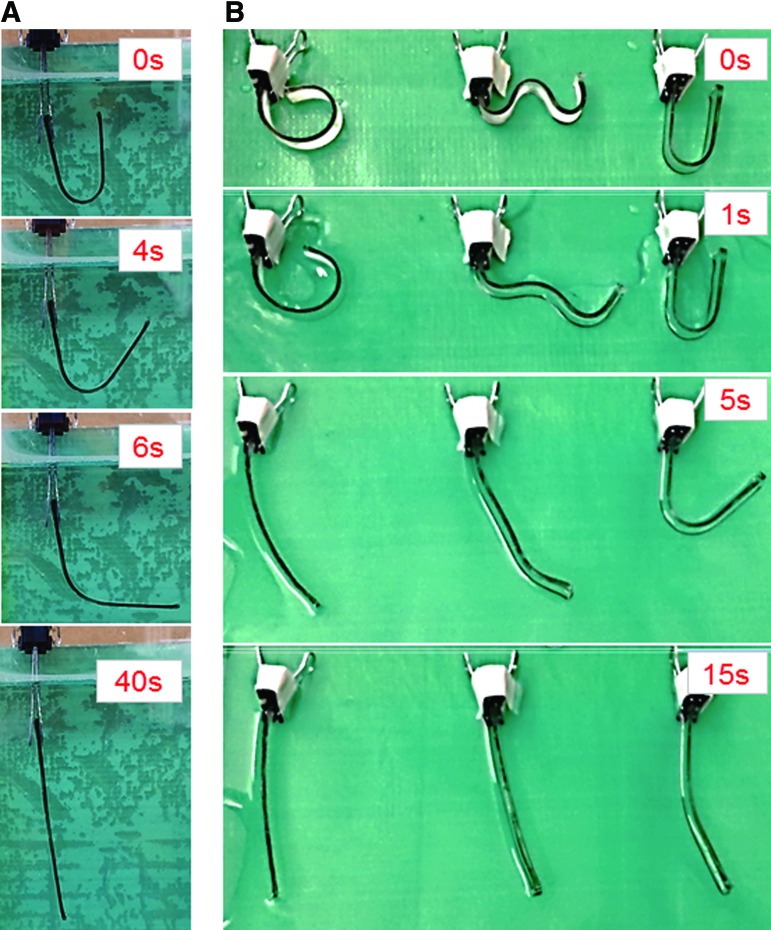
Shape memory effects were evaluated at a recovery temperature of 37°C, based on the intended application of synthesized smart polymers as potential implantable biomaterials. The shape recovery process is illustrated in Figure 7. As shown in Figure 7A, sample C40P300PH fully recovers from a programmed “U” shape to its original rectangular shape in 40 s with other smart polymer mixtures displaying recovery speeds ranging from 3.9 to 75.5°/s. As shown in Figure 7B, samples C80P300PH, C40P300HD, and C40P300PH are fixed at −18°C to “GWU” and restore their permanent shape at various speeds. Sample C40P300PH exhibits an obvious delay in recovery speed when compared to the other two samples.
FIG. 7.
The demonstration of the shape memory effects of the synthesized smart polymers: (A) sample C40P300PH was fixed at 0°C and recovered at 37°C; (B) samples C80P300PH, C40P300HD, and C40P300PH were fixed as “GWU” at −18°C and recovered at 37°C with different recovery speed. Color images available online at www.liebertpub.com/tec
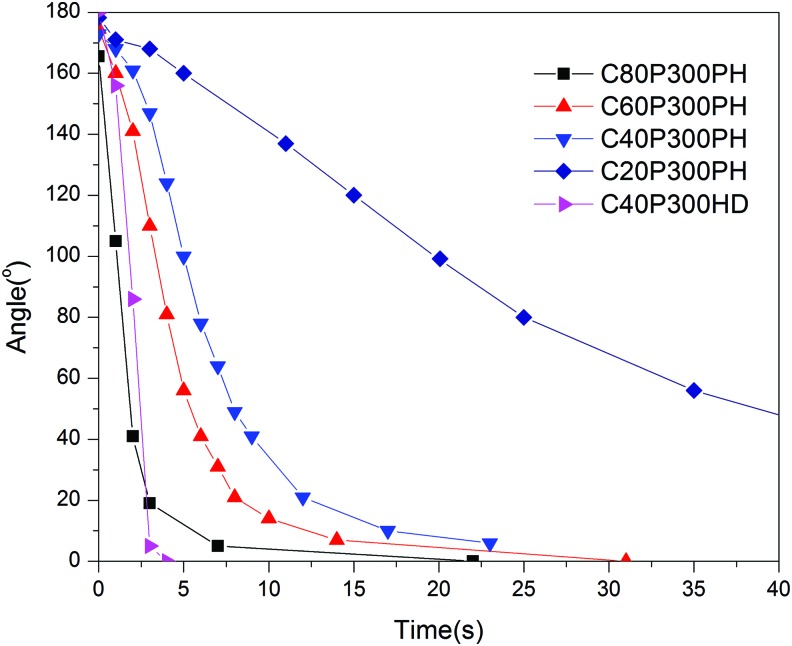
The quantitative recovery speed can be seen in Figure 8. Full recovery is achieved when the sample recovers its original linear rectangular shape with a bend angle of 0°. Nearly all the samples recover their original shape within 30 s, while sample C20P300PH requires ∼3 min to completely recover. The detailed shape memory effects of the smart polymers are found in Table 2 with all exhibiting excellent shape fixity, shape recovery, and widely adjustable properties with simple alterations in polymer composition.
FIG. 8.
Recovery curves of the synthesized smart polymers that were fixed at −18°C for a temporary shape and recovered at 37°C to their permanent shape. Color images available online at www.liebertpub.com/tec
Table 2.
Shape Memory Effects of the Synthesized Smart Polymers
| Sample code | Tgc (°C) | Rf (%) | Rr (%) | Recovery speed (°/s)a |
|---|---|---|---|---|
| C80P300PH | −8 | 92 | 100 | 69.5 |
| C60P300PH | 9 | 97 | 100 | 30.0 |
| C40P300PH | 21 | 96 | 100 | 23.1 |
| C20P300PH | 35 | 99 | 100 | 3.9 |
| C40P300HD | 0 | 100 | 100 | 75.5 |
Recovery speed, the fastest recovery speed during the recovering.
Fabrication of biomimetic scaffold
With a 3D printed PLA scaffold serving as a sacrificial mold, a biomimetic gradient structure was readily obtained with the synthesized smart polymers. Alterations in porosity was achieved by modifying the layer infill density as described in our previous work where fused deposition modeling (FDM) printing parameters of the sacrificial mold were correlated to the resultant structure.25 A greater infill density leads to a tighter printed lattice resulting in a more porous layer after dissolution of PLA.
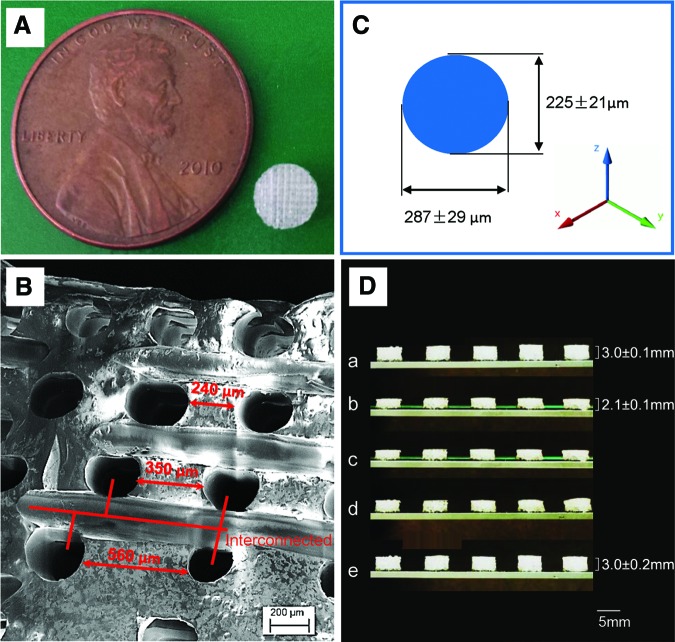
As shown in Figure 9A, a porous scaffold with a diameter of 5 mm was fabricated. The pores have a gradient distribution from the top to the bottom as the distance between pores increases from 240 to 560 μm, as shown in Figure 9B. This type of gradient porosity mimics the gradient porosity in natural tissues. In addition, the pores exhibit excellent interconnectivity as seen in Figure 9B, allowing for good nutrient perfusion and waste removal. The diameter of the pores is ∼250 μm, which can be readily altered by changing the 3D printer nozzle diameter. Pore size distribution is displayed in Figure 9C.
FIG. 9.
The fabricated scaffold. (A) A 5-mm-diameter and 3-mm-thickness scaffold compared to a cent. (B) The SEM image of the pore distribution in the scaffold. (C) Varied pore diameters in different directions. (D) The potential for minimally invasive application; a, sample original shape; b, temporary shape at −18°C; c, 0 s at 37°C; d, 10 s at 37°C; e, 3 min at 37°C; from left to right, the samples are C80P300PH, C60P300PH, C40P300PH, C20P300PH, and C40P300HD, respectively. Color images available online at www.liebertpub.com/tec
Interestingly, the pore diameter measured in the z-axis is ∼225 μm, while roughly 287 μm in x and y directions. This may be due to deformation of the PLA filament on extrusion through the high-temperature nozzle where PLA is affected by gravity as well as pressure from the flowing material for interlayer adhesion resulting in pore diameter variance. The possibility of using this system in a minimally invasive surgical procedure was evaluated (Fig. 9D); the fabricated implantable scaffold can be compacted to a thickness of ∼70% of original height (from 3.0 to 2.1 mm) and can be fully recovered when the scaffold is exposed to physiological temperature.
Bioevaluation with MSC
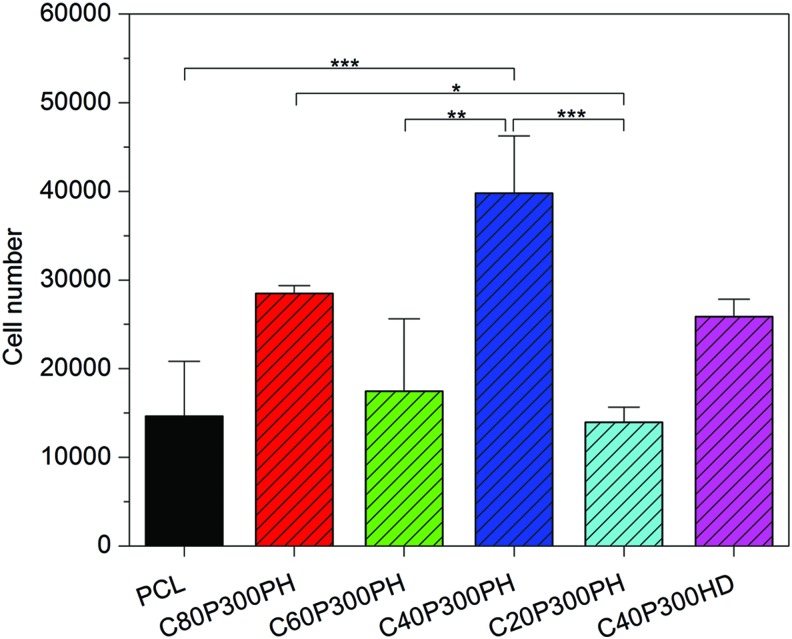
Figure 10 shows the results of 4-h MSC attachment on PCL control and synthesized smart polymers, illustrating that the smart polymers exhibit similar or greater cell attachment when compared to control. Particularly, sample C40P300PH has the highest cell attachment density exhibiting a 1.7-, 1.3-, and 1.8-fold increase when compared to PCL control, C60P300PH, and C20P300PH, respectively. In addition, C80P300PH has significantly better cell attachment than C20P300PH, about a onefold increase.
FIG. 10.
Four-hour adhesion of MSCs on the synthesized smart polymers. Data are mean ± standard deviation, n = 6. *p < 0.05, **p < 0.01, and ***p < 0.001. MSCs, mesenchymal stem cells. Color images available online at www.liebertpub.com/tec
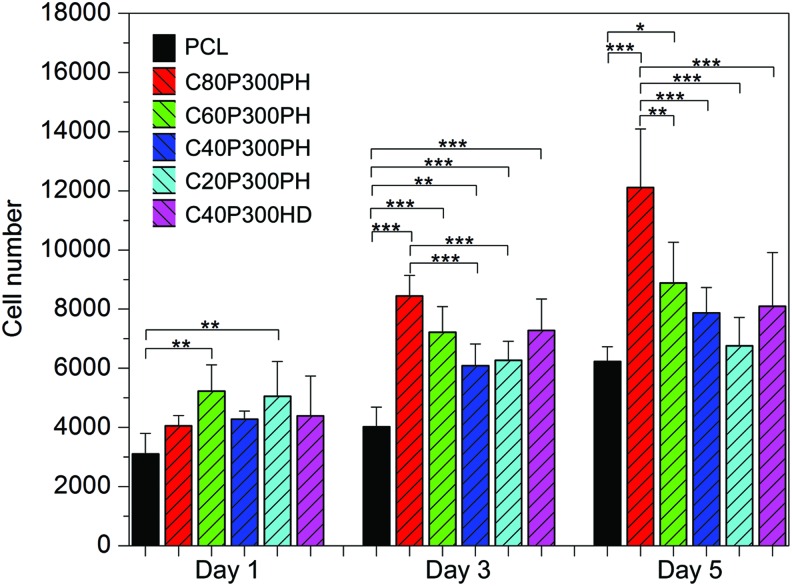
Figure 11 shows MSC proliferation after 1-, 3-, and 5-day culture on PCL control and the smart polymers. All the samples show excellent MSC proliferation at all time points. Specifically, sample C80P300PH has the highest MSC density with a 110% and 90% increase of cell density when compared to PCL control after 3 and 5 days, respectively. All smart polymers showed significantly higher proliferation when compared to PCL control after 3 days. Another interesting phenomenon is that MSC proliferation increases with increasing castor oil content as seen after 3 and 5 days (Fig. 11). For example, C80P300PH elicited a 35% and 79% increase in MSC density when compared to C20P300PH after 3 and 5 days, respectively.
FIG. 11.
One-, 3-, and 5-day proliferation of MSCs on the synthesized smart polymers. Data are mean ± standard deviation, n = 6. *p < 0.05, **p < 0.01, and ***p < 0.001. Color images available online at www.liebertpub.com/tec
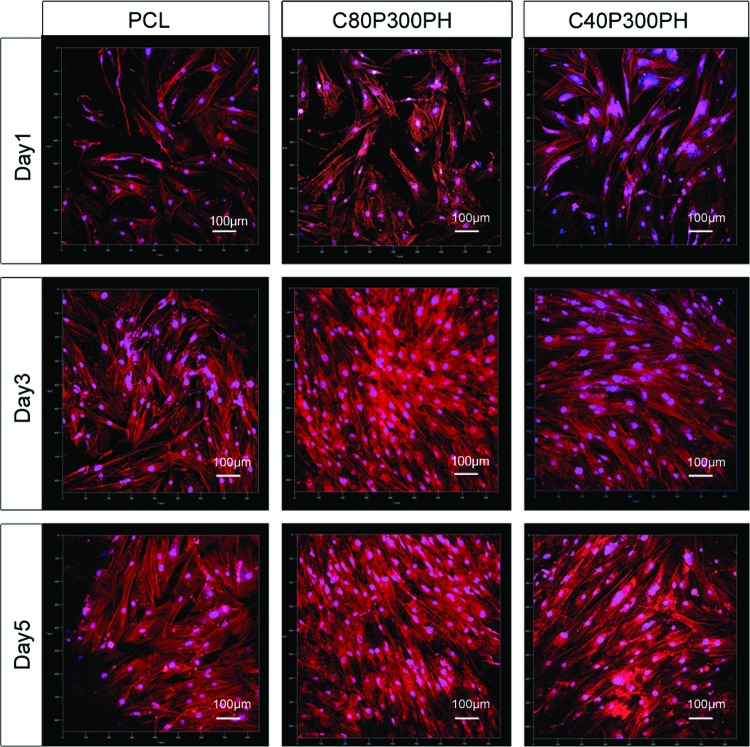
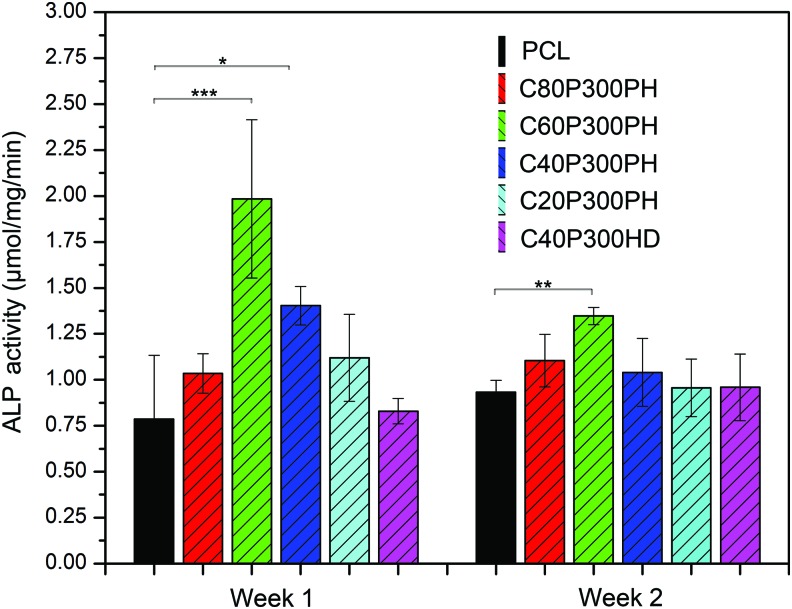
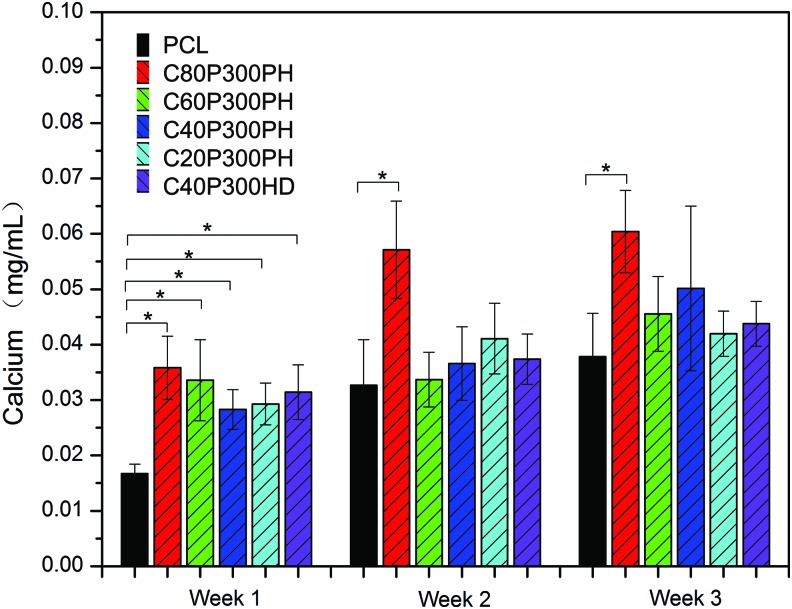
Increased cell proliferation was qualitatively evaluated by confocal analysis as shown in Figure 12. Both C80P300PH and C40P300PH exhibited excellent cell spreading morphology and increased cell growth density when compared to PCL control. Figure 13 shows the ALP activity of MSC differentiation on various sample groups. The samples C60P300PH and C40P300PH showed significantly higher ALP activity than PCL control on week 1; only sample C60P300PH showed greater ALP-specific activity when compared to PCL control on week 2. Figure 14 shows the calcium deposition on different materials. All the smart materials exhibited a significant higher calcium deposition than PCL control on week 1; only sample C40P300PH had greater calcium deposition than PCL control on weeks 2 and 3.
FIG. 12.
Confocal microscopy images of MSC growth and spreading morphology on C40P300PH and C20P300PH when compared with PCL control after 1-, 3-, and 5-day culture. The color red represents cell cytoskeleton and the color blue represents cell nuclei. Color images available online at www.liebertpub.com/tec
FIG. 13.
ALP activity on different synthesized smart polymers compared to PCL control. Data are mean ± standard deviation, n = 6. *p < 0.05, **p < 0.01, and ***p < 0.001. ALP, alkaline phosphatase. Color images available online at www.liebertpub.com/tec
FIG. 14.
Enhanced total calcium deposition on smart polymers compared to PCL control. Data are mean ± standard deviation, n = 6. *p < 0.05. Color images available online at www.liebertpub.com/tec
Discussion
The shape memory functionality of synthesized smart polymers is highly dependent on the material composition. Although both Ptriol300 and Ptriol900 were utilized to synthesize the polymeric networks, only Ptriol300-based samples display a shape memory effect. Both HD and PH are capable of fully crosslinking the polymers where gel contents of the polymeric networks are greater than 95%. Among the 22 synthesized polymers, five are capable of temporary shape fixation at 0°C or −18°C with four of the five using PH as the crosslinking agent. In the polymeric networks, the crosslinking net points are intended to maintain the original shape, while the glass transitions provide the mechanism for temporary shape fixation. The networks based on Ptriol300 and PH provide a suitable glass transition to perform the shape fixity effect under the intended test conditions.
Uniaxial compression tests indicate the presence of interpenetrating polymeric networks (IPNs). Two networks are present in the synthesized smart polymers: network I (castor oil+PH) and network II (Ptriol300+PH). When the weight ratio of castor oil to Ptriol300 is greater than 40:60, network I is dominant; when the ratio is lower than 40:60, network II is the main crosslinking structure; in sample C40P300PH, networks I and II are both fully crosslinked, resulting in a complete IPN. It is a typical phenomenon that IPNs can exhibit enhanced properties than both substituent polymers alone due to inter net-locking structures.26,27 Similar results are observed when HD is used as the crosslinker. The compression modulus of sample C40P300HD is significantly higher than others although Ptriol300HD is not formed due to reagent immiscibility.
The IPN structure can also be used to interpret DSC results. Network I tends to exhibit a lower Tg, while network II tends to shift the Tg to a higher temperature. For example, sample C40P300PH exhibits full crosslinking of both networks I and II, which has a tendency to keep both glass transitions and results in a broader Tg breadth.
Sample C40P300PH exhibits significantly higher MSC attachment when compared to PCL control. The attachment of MSCs is affected by multiple parameters, including surface hydrophobicity, surface morphology, material toughness, and chemical composition. Water contact angle analysis suggests that the hydrophobicity of the smart polymers is not statistically different than PCL. SEM analysis shows that there is no significant difference between sample C40P300PH surface and other sample surfaces. Sample C40P300PH shows a higher compression modulus than other smart polymer samples but is close to that of PCL. Taken collectively, it can be postulated that the increased cellular attachment is chemically mediated by the composition of the newly synthesized smart polymers. In addition to the presence of Ptriol300 segments structurally similar to PCL, the smart polymers contain urea and urethane groups (Fig. 2), which may contribute to improved MSC attachment.28
The smart polymers with higher castor oil content show significantly higher MSC proliferation when compared to PCL control and smart polymers with lower castor oil content at 3 and 5 days. The greater MSC proliferation may be attributed to the combined effects of mechanical and chemical properties of the samples as described above. An interesting observation was made when samples containing a higher content of castor oil (higher content of polymeric network I) leading to greater MSC proliferation. Sample C80P300PH shows the highest proliferation rate, with this sample having more castor oil than other smart polymers and PCL control. Therefore, MSCs may prefer a predominant castor oil network. These findings lend themselves to further studies of copolymers from PCL and plant oils for smart tissue applications.
ALP is a known in vitro osteogenic differentiation marker.29 The increase of ALP activity on the synthesized smart polymers indicates their great potential as bone regenerative materials. Sample C60P300PH showed significantly higher ALP activity than PCL control on both weeks 1 and 2. The enhanced MSC differentiation on weeks 1, 2, and 3 is further confirmed by calcium deposition. Similar to MSC proliferation, the enhanced MSC differentiation, even early on weeks 1 and 2, may be attributed to the combined effects of mechanical and chemical properties. The effect of plant oil residue within the polymer may be of particular interest because sample C80P300PH exhibited the highest calcium deposition in all weeks and this sample had the highest castor oil content, which may lead to a greater interest in plant oil-based materials for biomedical applications.
The 4D printing technique used here will further advance the application of 3D technology in fabricating shape memory scaffolds with thermosetting polymers. 3D printing technologies are vastly increasing in popularity due to the ability to directly print porous scaffolds with designed shape and interconnected porosity from a CAD file with a variety of materials such as ceramic, metallic, polymeric, and composite. Biopolymers are particularly important due to their excellent biocompatibility and functionality.
Among 3D printing techniques, FDM is one of the most applied and commercialized technologies.30,31 Typically, a thermoplastic filament material is forced out of a temperature-controlled extruder, and the molten polymer is deposited on a platform layer by layer. However, thermosetting polymers cannot be melted and reshaped after they are cured, very different from traditional 3D printable thermoplastic polymers.32 Therefore, thermosetting polymers are largely incompatible with FDM-based printers.33
Instead, fully controlled gradient scaffolds can be fabricated by curing thermosetting polymers with FDM printed sacrificial molds as demonstrated in this study. Another advantage of this guided approach is in providing different pore morphologies. Generally, direct 3D printing techniques contain inherent difficulties to form small-sized tubular channels around 250 μm; the pores in the FDM printed scaffold from the guided approach here are actually the shape of the extruded fiber, which can be readily adjusted by shaping the size and geometry of the nozzle.34,35 The channel-like pore structure may provide better conditions for vascularization in view of the similarity between tubular channels and blood vessels, which extends beyond the scope of this study.
Conclusion
The synthesized smart polymers, which have close compression modulus and surface hydrophobicity to PCL, exhibit excellent shape memory effects with various recovery speeds at physiological temperature from a fixed temporary shape. When combined with a sacrificial 3D printed PLA mold, scaffolds of graded porosity and shape memory effect can be readily fabricated, which provides a facile method for developing complex graded scaffolds for tissue engineering applications. This 4D printing technique not only provides gradient distributed channel morphology but also illustrates the great potential of 4D technology in developing scaffolds from thermosetting polymers, which are not printable with FDM-based 3D printers. All the smart polymers exhibit excellent attachment, proliferation, and differentiation of MSCs. The excellent shape memory effect, extraordinary cytocompatibility, and complex graded structure illustrate the great potential for regenerative medicine applications.
Acknowledgments
The authors thank NIH Director's New Innovator Award (DP2EB020549) and March of Dimes Foundation's Gene Discovery and Translational Research Grant for financial support. They thank Dr. Xinran Zhang, Assistant Director of the Institute for Soft Matter Synthesis and Metrology at Georgetown University, for DSC analysis.
Disclosure Statement
No competing financial interests exist.
References
- 1.Dhandayuthapani B., Yoshida Y., Maekawa T., and Kumar D.S. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci 2011, 1, 2011 [Google Scholar]
- 2.Wauthle R., Van der Stok J., Yavari S.A., Van Humbeeck J., Kruth J.P., Zadpoor A.A., Weinans H., Mulier M., and Schrooten J. Additively manufactured porous tantalum implants. Acta Biomater 14, 217, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Seol Y.J., Park J.Y., Jung J.W., Jang J., Girdhari R., Kim S.W., and Cho D.W. Improvement of bone regeneration capability of ceramic scaffolds by accelerated release of their calcium ions. Tissue Eng Part A 20, 2840, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cipitria A., Reichert J.C., Epari D.R., Saifzadeh S., Berner A., Schell H., Mehta M., Schuetz M.A., Duda G.N., and Hutmacher D.W. Polycaprolactone scaffold and reduced rhBMP-7 dose for the regeneration of critical-sized defects in sheep tibiae. Biomaterials 34, 9960, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Thadavirul N., Pavasant P., and Supaphol P. Development of polycaprolactone porous scaffolds by combining solvent casting, particulate leaching, and polymer leaching techniques for bone tissue engineering. J Biomed Mater Res A 102, 3379, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Miao S., Callow N.V., and Ju L.K. Ethyl rhamnolipids as a renewable source to produce biopolyurethanes. Eur J Lipid Sci Tech 117, 156, 2015 [Google Scholar]
- 7.Jensen J., Rölfing J.H.D., Le S., Quang D., Kristiansen A.A., Nygaard J.V., Hokland L.B., Bendtsen M., Kassem M., and Lysdahl H. Surface-modified functionalized polycaprolactone scaffolds for bone repair: in vitro and in vivo experiments. J Biomed Mater Res A 102, 2993, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Becker J., Lu L., Runge M.B., Zeng H., Yaszemski M.J., and Dadsetan M. Nanocomposite bone scaffolds based on biodegradable polymers and hydroxyapatite. J Biomed Mater Res A 103, 2549, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Zhang D., George O.J., Petersen K.M., Jimenez-Vergara A.C., Hahn M.S., and Grunlan M.A. A bioactive “self-fitting” shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomater 10, 4597, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Machado L., and Savi M. Medical applications of shape memory alloys. Braz J Med Biol Res 36, 683, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Seol Y.J., Kang T.Y., and Cho D.W. Solid freeform fabrication technology applied to tissue engineering with various biomaterials. Soft Matter 8, 1730, 2012 [Google Scholar]
- 12.Phipps M.C., Clem W.C., Grunda J.M., Clines G.A., and Bellis S.L. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 33, 524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akbarzadeh R., and Yousefi A.M. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater 102, 1304, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Intranuovo F., Gristina R., Brun F., Mohammadi S., Ceccone G., Sardella E., Rossi F., Tromba G., and Favia P. Plasma modification of PCL porous scaffolds fabricated by solvent-casting/particulate-leaching for tissue engineering. Plasma Process Polym 11, 184, 2014 [Google Scholar]
- 15.Jabbarzadeh E., Deng M., Lv Q., Jiang T., Khan Y.M., Nair L.S., and Laurencin C.T. VEGF-incorporated biomimetic poly (lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater 100, 2187, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Suárez-González D., Barnhart K., Migneco F., Flanagan C., Hollister S.J., and Murphy W.L. Controllable mineral coatings on PCL scaffolds as carriers for growth factor release. Biomaterials 33, 713, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inzana J.A., Olvera D., Fuller S.M., Kelly J.P., Graeve O.A., Schwarz E.M., Kates S.L., and Awad H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 35, 4026, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bose S., Vahabzadeh S., and Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today 16, 496, 2013 [Google Scholar]
- 19.Murphy S.V., and Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 32, 773, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Tibbits S. 4D printing: Multi-material shape change. Archit Des 84, 116, 2014 [Google Scholar]
- 21.Ge Q., Qi H.J., and Dunn M.L. Active materials by four-dimension printing. Appl Phys Lett 103, 131901, 2013 [Google Scholar]
- 22.Kylmä J., and Seppälä J.V. Synthesis and characterization of a biodegradable thermoplastic poly (ester-urethane) elastomer. Macromolecules 30, 2876, 1997 [Google Scholar]
- 23.Liu C., Chun S.B., Mather P.T., Zheng L., Haley E.H., and Coughlin E.B. Chemically cross-linked polycyclooctene: synthesis, characterization, and shape memory behavior. Macromolecules 35, 9868, 2002 [Google Scholar]
- 24.Cai K., Yao K., Lin S., Yang Z., Li X., Xie H., Qing T., and Gao L. Poly (D, L-lactic acid) surfaces modified by silk fibroin: effects on the culture of osteoblast in vitro. Biomaterials 23, 1153, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Castro N., Patel R., and Zhang L. Design of a novel 3D printed bioactive nanocomposite scaffold for improved osteochondral regeneration. Cell Mol Bioeng 8, 416, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ha S.M., Yuan W., Pei Q., Pelrine R., and Stanford S. Interpenetrating polymer networks for high-performance electroelastomer artificial muscles. Adv Mater 18, 887, 2006 [Google Scholar]
- 27.Waters D.J., Engberg K., Parke-Houben R., Ta C.N., Jackson A.J., Toney M.F., and Frank C.W. Structure and mechanism of strength enhancement in interpenetrating polymer network hydrogels. Macromolecules 44, 5776, 2011 [Google Scholar]
- 28.Miao S., Sun L., Wang P., Liu R., Su Z., and Zhang S. Soybean oil-based polyurethane networks as candidate biomaterials: synthesis and biocompatibility. Eur J Lipid Sci Tech 114, 1165, 2012 [Google Scholar]
- 29.Haversath M., Hülsen T., Böge C., Tassemeier T., Landgraeber S., Herten M., Warwas S., Krauspe R., and Jäger M. Osteogenic differentiation and proliferation of bone marrow-derived mesenchymal stromal cells on PDLLA+ BMP-2-coated titanium alloy surfaces. J Biomed Mater Res A 104, 145, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Zein I., Hutmacher D.W., Tan K.C., and Teoh S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 23, 1169, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Meakin J., Shepherd D., and Hukins D. Fused deposition models from CT scans. Br J Radiol 77, 504, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Pascault J.P., Sautereau H., Verdu J., and Williams R.J. Thermosetting polymers. CRC Press, New York, 2002 [Google Scholar]
- 33.Wong K.V., and Hernandez A. A review of additive manufacturing. ISRN Mechanical Engineering 2012, 1, 2012 [Google Scholar]
- 34.Meyer W., Engelhardt S., Novosel E., Elling B., Wegener M., and Krüger H. Soft polymers for building up small and smallest blood supplying systems by stereolithography. J Funct Biomater 3, 257, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis J.A. Direct ink writing of 3D functional materials. Adv Funct Mater 16, 2193, 2006 [Google Scholar]