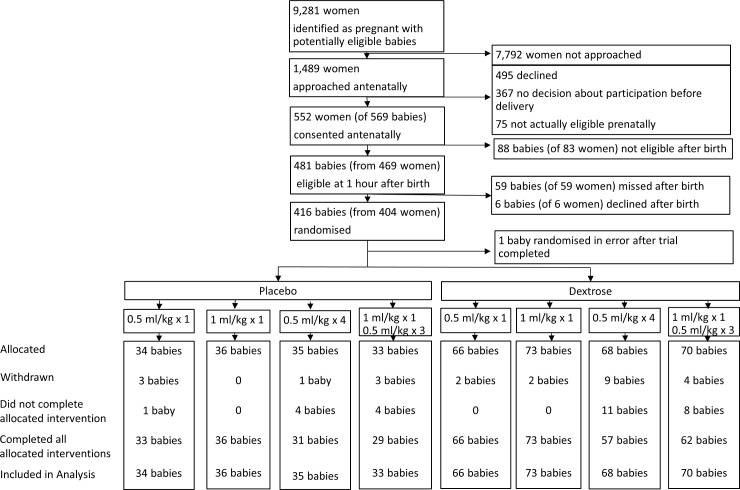
Fig 1. Consolidated Standards of Reporting Trials (CONSORT) diagram.
One baby was randomised in error following closure of the trial after randomisation of 415 babies and was excluded from the analysis. All other babies had primary outcome data available and were included in the intention-to-treat analysis.