Abstract
Background and Aims
Understanding contributions of lean and fat tissue to cardiovascular and non-cardiovascular mortality may help clarify areas of prevention in older adults. We aimed to define distributions of lean and fat tissue in older adults and their contributions to cause-specific mortality.
Methods and Results
A total of 1335 participants of the Cardiovascular Health Study (CHS) who underwent dual-energy x-ray absorptiometry (DEXA) scans were included. We used principal components analysis (PCA) to define two independent sources of variation in DEXA-derived body composition, corresponding to principal components composed of lean (“lean PC”) and fat (“fat PC”) tissue. We used Cox proportional hazards regression using these PCs to investigate the relationship between body composition with cardiovascular and non-cardiovascular mortality. Mean age was 76.2±4.8 years (56% women) with mean body mass index 27.1±4.4 kg/m2. A greater lean PC was associated with lower all-cause (HR=0.91, 95% CI 0.84-0.98, P=0.01) and cardiovascular mortality (HR=0.84, 95% CI 0.74-0.95, P=0.005). The lowest quartile of the fat PC (least adiposity) was associated with a greater hazard of all-cause mortality (HR = 1.24, 95% CI 1.04-1.48, P=0.02) relative to fat PCs between the 25th-75th percentile, but the highest quartile did not have a significantly greater hazard (P=0.70).
Conclusion
Greater lean tissue mass is associated with improved cardiovascular and overall mortality in the elderly. The lowest levels of fat tissue mass are linked with adverse prognosis, but the highest levels show no significant mortality protection. Prevention efforts in the elderly frail may be best targeted toward improvements in lean muscle mass.
Keywords: DEXA, fat, lean mass, cardiovascular mortality, all-cause mortality, Cardiovascular Health Study
Introduction
The association between body tissue composition and mortality in older adults (>65 years of age) is controversial. While overweight BMI is associated with improved outcomes[1], a “protective” effect of obesity on adverse health outcomes may be attenuated with increasing age[2]. Unlike results in younger populations, studies in the elderly have found a complex, inconsistent relationship between mortality, BMI and waist circumference [3]. Moreover, the few large population studies that employ tissue quantification using dual energy x-ray absorptiometry (DEXA) or tomographic imaging[4-8] have yielded conflicting associations between lean or fat mass and clinical outcome. Hence, the impact of lean and fat tissue on cardiovascular and overall mortality remains an open question in older adults[9]. Clarification of the relative contribution of lean and fat tissue to cardiovascular and non-cardiovascular mortality in large, well-characterized aging cohorts may shed additional light on areas for prevention and intervention to maintain favorable body composition.
In participants of the Cardiovascular Health Study (CHS), one of the largest population-based samples of older Americans, we used DEXA imaging to quantify lean and fat tissue and measured their association with overall and cause-specific mortality. Given the variety of DEXA-derived lean and fat mass indices used previously in this field and the high correlation between these indices, we used data reduction with principal components analysis (PCA) to derive independent body composition signatures. We compared the association of these signatures, and aggregate DEXA-derived total lean, appendicular lean mass, total fat and trunk fat mass with cardiovascular and all-cause mortality. Given the distinct biological roles of lean tissue and adiposity[9-12], we hypothesized that decreased lean mass and greater fat in an elderly population would be associated with an increased risk of mortality.
Methods
Study design and population
The Cardiovascular Health study (CHS) is a population-based cohort study of cardiovascular risk factors in adults 65 years or older recruited from 4 communities in the United States (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania). The study design was previously published[13, 14]. An original cohort was recruited in 1989-1990 and a second, predominantly African American cohort, was recruited in 1992-1993. Clinic examinations were performed annually from 1989-1990 through 1998-1999, and again in 2005–2006. We used adjudicated events follow-up data completed as of June 30, 2013. Institutional Review Boards at each site and the coordinating center approved the study methods. All participants provided written informed consent.
Our study focused on 1575 participants undergoing DEXA scans during the 1994-1995 examination. DEXA was performed at 2 study sites (Sacramento County and Allegheny County), as part of a nested study on body composition[15]. To limit confounding of our associations between regional tissue composition and outcome by undetermined illness or frailty, we excluded participants with ≥5% weight loss (n=193) from 1992-1993 to 1994-1995 or with missing weight or height data (n=47), leaving 1335 participants in our final analytic sample.
Detailed clinical data including anthropometric indices, laboratory and imaging data have been collected as described[13]. Weight was measured using a calibrated balance beam scale. Standing height was measured in centimeters using a calibrated stadiometer. Waist circumference was measured at the level of the umbilicus. BMI was calculated from the most recently available weight and height. Physical activity was calculated using the Minnesota Leisure Time Activities questionnaire as kilocalories per week. Cystatin C-based glomerular filtration rate (eGFRcys) was calculated using the equation eGFRcys =76.7*cystatinC-1.19. Hypertension was defined as a systolic blood pressure of ≥140 mmHg, a diastolic blood pressure ≥ 90 mmHg, or use of antihypertensive medications. Diabetes was defined as a fasting glucose ≥126mg/dl, non-fasting glucose ≥200mg/dl or the use of diabetes medications. Prevalent coronary heart disease (CHD: angina, myocardial infarction, coronary bypass or angioplasty), congestive heart failure (CHF), stroke/transient ischemic attack (TIA) and peripheral vascular disease (PVD) were adjudicated using standardized criteria[14]. Atrial fibrillation (AF) was determined by 12-lead electrocardiogram (ECG).
End stage renal disease (ESRD) was defined as renal failure requiring renal replacement therapy. Prevalent cancer and liver disease were determined from self-reported physician diagnoses. Chronic obstructive pulmonary disease (COPD) was defined as self-reported physician diagnosis of chronic bronchitis, asthma or emphysema and spirometry data. Predicted values of FEV1 and FVC were obtained from prediction equations derived from the Third National Health and Nutrition Examination Survey[16]. Severe COPD was defined as Global Initiative on Obstructive Lung Disease classification criteria stage 3 or 4 COPD (FEV1/FVC < 0.70 and FEV1 < 50% predicted)[17, 18]. Covariates were measured at the 1994-1995 examination and those not measured at the 1994-1995 visit were obtained from prior examinations (Supplemental Table S1).
Dexa
Dual-energy x-ray absorptiometry measurements were performed using QDR-2000 bone densitometers (Hologic, Inc., Bedford, Massachusetts)[15] using array beam mode. Whole-body DEXA data was used to calculate total mass, lean and fat mass with QDR software version 7.10 (Hologic, Bedford, MA). Lean mass values do not include bone mineral content. Scans were read blindly and were monitored for quality control by the University of California, San Francisco reading center. All DEXA-derived indices were normalized to height2, as previously performed in other large cohort studies[9]. Appendicular lean mass was defined as the sum of right and left arm and leg lean mass divided by height2.
Clinical endpoints
Our primary endpoint was all-cause mortality. Non-cardiovascular death (defined as any death not definitely of cardiovascular origin) and cardiovascular death were secondary endpoints. Cardiovascular death was defined as death from atherosclerotic coronary artery disease, cerebrovascular disease, other atherosclerotic disease and other cardiovascular illnesses, which included but were not limited to those corresponding to the International Classification of Diseases, Ninth revision (ICD-9) codes for rheumatic heart disease, hypertensive disease, pulmonary heart disease, pericarditis, endocarditis, myocarditis, dysrhythmias, conduction disorders, heart failure, peripheral arterial disease, aortic aneurysm or dissection and venous thromboembolism[14]. Methods for ascertainment of deaths have been previously described.[14] In brief, mortality surveillance was prospectively conducted at each field center every 6 months, and deaths were ascertained by proxy report, local obituaries, Social Security Death Index searches, searches of Medicare claims data and review of medical records. All deaths were centrally adjudicated by a panel of physicians using standardized criteria[14].
Statistical analysis
Baseline characteristics were expressed as mean and standard deviation, median and interquartile range or number (percent) with comparisons made by appropriate parametric or non-parametric testing (based on data normality). We estimated Spearman correlation coefficients (adjusted for sex) to measure the association among DEXA-derived tissue composition measures, BMI and waist circumference (Supplemental Table S2).
Given the high correlation between DEXA-derived tissue composition measures and expected variability in tissue composition by sex, we next performed a sex-specific principal component analysis (PCA) of lean and fat DEXA components: total lean and fat mass, trunk lean and fat mass, right/left arm/leg lean and fat mass (each adjusted by height2). We calculated sex-specific lean and fat PCs as the linear combination of PC weightings and standardized DEXA tissue components. Male and female PCs were pooled and used as continuous variables in regression.
For survival analysis, follow-up time was defined in participant-days from the date of the DEXA scan to the date of death or the end of follow-up. Given prior conflicting reports of the form of the relationship between tissue distributions and mortality, we chose to investigate the form of the relationship between mortality and lean PC, fat PC, and selected DEXA-derived tissue components previously used in other studies (total lean, appendicular lean, total fat and trunk fat mass) using restricted cubic spline Cox regression (macro RCS[19]). We used 5 knots fitted to the central 95% of the observations. The Wald test for non-linearity was performed as described in previous work[20, 21]. We used piecewise quadratic models to further investigate the relationship of the fat PC with mortality.
We estimated Cox proportional hazards models for all-cause and cause-specific (cardiovascular and non-cardiovascular) mortality. Given that lean and fat PCs are independent (by construction in PCA), we constructed one model including both PCs as primary predictors of interest. In sensitivity analyses, we constructed separate Cox models to specify the association between outcomes and height indexed total lean mass, appendicular lean mass, total fat mass or trunk fat mass. Fat PC was modeled in quartiles, with the middle two quartiles (25th-75th percentile) as the reference category corresponding to the visual lowest log hazard ratio in adjusted splines. All other DEXA components were modeled linearly. Models were adjusted for age, sex, race (African-American vs. non-African-American), education (completion of grade 12 education, graduate equivalency degree or beyond vs. less than grade 12 education), income (≥$25,000 vs. < $25,000), smoking (ever smoker vs. non-smoker), leisure-time activity (above vs. below median kilocalories per week), eGFRcys (ml/min/1.73m2), presence of hypertension, presence of diabetes, and any prior cardiovascular disease (composite of CHD, stroke or TIA, CHF, PVD and AF). Multiplicative interaction terms with sex and race were examined in each model. Finally, because of the concern for residual confounding or bias introduced by smoking and chronic illness, we performed three additional sensitivity analyses: (1) excluding CHS participants with chronic illnesses (N=273; history of liver disease, cancer, ESRD or severe COPD)[22]; (2) excluding current smokers (N=108); (3) censoring mortality events that occurred up to 1 year from the date of the DEXA scan. All continuous variables were standardized for regression analyses.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). A two-tailed P value of 0.05 was considered significant.
Results
Baseline clinical characteristics and DEXA-derived indices
Demographic, clinical and DEXA information stratified by sex are presented in Table 1. In our analytic cohort, 56% were women, and 18% were African-American (mean age 76.2±4.8 years). Mean BMI was 27.1±4.4 kg/m2. DEXA-derived fat indices were greater in women, while lean indices were greater in men (Table 1).
Table 1.
Baseline characteristics of 1335 CHS participants who underwent DEXA.
| Overall | Male | Female | |
|---|---|---|---|
| n ≤ 1335 | n ≤ 582 | n ≤ 753 | |
| Age, mean (SD), y | 76.2 ± 4.8 | 76.7 ± 5.0 | 75.8 ± 4.5 |
| African American, n (%) | 243 (18.2) | 100 (17.2) | 143 (19.0) |
| Highschool graduate or GED and beyond, n (%) | 1122 (84.1) | 490 (84.3) | 632 (83.9) |
| Income ≥$25,000, n (%)* | 569 (50.3) | 298 (60.0) | 271 (42.7) |
| Ever smoker, n (%) | 763 (57.3) | 403 (69.4) | 360 (48.0) |
| Leisure-time activity in kcal/week, median (IQR) | 981.2 (349.4, 2165.6) | 1335.0 (540.0, 2732.5) | 810.0 (270.0, 1807.5) |
| Weight, mean (SD), kg | 73.5 ± 13.9 | 80.1 ± 11.7 | 68.5 ± 13.4 |
| Height, mean (SD), cm | 164.6 ± 9.5 | 172.4 ± 6.6 | 158.6 ± 6.5 |
| BMI, mean (SD), kg/m2 | 27.1 ± 4.4 | 26.9 ± 3.7 | 27.2 ± 4.9 |
| Waist circumference, mean (SD), cm | 96.1 ± 12.7 | 98.2 ± 9.7 | 94.5 ± 14.4 |
| eGFRcys (ml/min/1.73m2) | 75.8 ± 18.3 | 71.9 ± 16.5 | 78.9 ± 19.0 |
| DEXA characteristics, mean (SD) | |||
| Height-indexed total fat mass, kg/m2 | 10.0 ± 3.9 | 7.9 ± 2.7 | 11.7 ± 3.8 |
| Height-indexed trunk fat mass, kg/m2 | 4.8 ± 2.0 | 4.1 ± 1.8 | 5.4 ± 2.0 |
| Height-indexed total lean mass, kg/m2 | 16.3 ± 2.5 | 18.2 ± 2.0 | 14.8 ± 1.8 |
| Height-indexed appendicular lean mass, kg/m2 | 6.6 ± 1.3 | 7.7 ± 1.0 | 5.8 ± 0.9 |
| Prevalent hypertension, n (%) | 742 (55.6) | 300 (51.6) | 442 (58.8) |
| Prevalent diabetes, n (%) | 194 (16.0) | 105 (20.3) | 89 (12.8) |
| Prevalent coronary heart disease, n (%) | 297 (22.3) | 173 (29.7) | 124 (16.5) |
| Prevalent heart failure, n (%) | 68 (5.1) | 41 (7.0) | 27 (3.6) |
| Prevalent stroke, n (%) | 56 (4.2) | 35 (6.0) | 21 (2.8) |
| Prevalent atrial fibrillation, n (%) | 42 (3.2) | 25 (4.4) | 17 (2.3) |
| Prevalent peripheral vascular disease, n (%) | 45 (3.4) | 33 (5.7) | 12 (1.6) |
| Prevalent end-stage renal disease, n (%) | 19 (1.4) | 12 (2.1) | 7 (0.9) |
| Prevalent liver disease, n (%) | 10 (0.8) | 5 (0.9) | 5 (0.7) |
| Prevalent GOLD Stage 3 or 4 COPD, n (%) | 30 (2.4) | 12 (2.2) | 18 (2.6) |
| Prevalent cancer, n (%) | 228 (17.1) | 102 (17.5) | 126 (16.7) |
Values are mean (standard deviation), median (interquartile range) or number (%). Abbreviations: BMI = body mass index; CHS = Cardiovascular Health Study; COPD = chronic obstructive pulmonary disease; DEXA = dual-energy x-ray absorptiometry; eGFRcys = cystatin C-based estimated glomerular filtration rate; GED = graduate equivalency degree; GOLD = Global Initiative for Obstructive Lung Disease; IQR = interquartile range; SD = standard deviation.
The greatest amount of missingness was in income with 204/1335 missing.
Principal components analysis of DEXA-derived regional tissue components
Given the strong interrelationships among DEXA-derived tissue metrics (Supplemental Table S2), we performed sex-stratified PCA on DEXA-derived tissue measures to define independent signatures of body composition. We defined 2 principal components (PCs) that explained 83.1% of the total variance in body composition in males and 83.9% in females (Table 2). Of note, DEXA lean tissue measures had a high loading in one PC (subsequently referred to as the “lean PC”), while the other PC was loaded more prominently on DEXA fat tissue measures (referred to as the “fat PC”).
Table 2.
Principal components analysis of DEXA lean and fat components. Values represent loadings for each DEXA component on a given principal component (PC).
| Male (n=582) | Female (n=753) | |||
|---|---|---|---|---|
| PC 1 Fat | PC 2 Lean | PC 1 Fat | PC 2 Lean | |
| Total fat mass | 0.96 | 0.15 | 0.96 | 0.24 |
| Total lean mass | 0.23 | 0.95 | 0.31 | 0.92 |
| Trunk fat mass | 0.89 | 0.10 | 0.86 | 0.25 |
| Trunk lean mass | 0.19 | 0.82 | 0.28 | 0.79 |
| Right arm fat mass | 0.88 | 0.16 | 0.87 | 0.29 |
| Right arm lean mass | 0.02 | 0.89 | 0.04 | 0.89 |
| Left arm fat mass | 0.89 | 0.17 | 0.86 | 0.30 |
| Left arm lean mass | 0.08 | 0.87 | 0.07 | 0.87 |
| Right leg fat mass | 0.90 | 0.20 | 0.92 | 0.12 |
| Right leg lean mass | 0.22 | 0.89 | 0.33 | 0.86 |
| Left leg fat mass | 0.91 | 0.19 | 0.92 | 0.10 |
| Left leg lean mass | 0.25 | 0.88 | 0.35 | 0.85 |
| Percentage of explained variance | 56.21 | 26.93 | 61.58 | 22.27 |
| Cumulative percentage of explained variance | 83.14 | 83.85 | ||
Lean and fat PCs are associated with clinical outcomes in older adults
Over a median follow-up of 12.0 years (interquartile range 7.2-17.5 years), 1047 (78.4%) deaths occurred, 382 (36.5%) cardiovascular and 665 (63.5%) non-cardiovascular in etiology. In spline models, lean PC was associated with mortality risk linearly; fat PC had a non-linear association with mortality in unadjusted but not adjusted models (Figure 1). Greater lean PC was associated with a lower all-cause (HR=0.91, 95% CI 0.84-0.98, P=0.01) and cardiovascular (HR=0.84, 95% CI 0.74-0.95, P=0.005) but not non-cardiovascular mortality (P=0.29, Table 3). Using piecewise quadratic models, fat PC <0 was associated with a non-linear increase in risk (p=0.0005 for quadratic), which was not true for fat PC >0 (P=0.85). Given the complex association of fat with mortality, we modeled fat PC in quartiles (25th-75th percentile as referent). The lowest quartile of fat PC was associated with greater all-cause mortality [hazard ratio (HR) = 1.24, 95% confidence interval (CI) 1.04-1.48, P=0.02] after full adjustment, with a trend for increased non-cardiovascular mortality (P=0.08, Table 3). The upper quartile of fat PC was not associated with all-cause or cause-specific mortality. As a comparison, we estimated all-cause and cause-specific mortality with raw DEXA components, with higher total and trunk fat as well as appendicular lean mass associated with decreased mortality (Table 4). There was no evidence that sex or race modified the association between the lean or fat PC and mortality. In sensitivity analyses restricted to those without prevalent smoking at the baseline visit or without chronic illnesses, associations were not substantially altered. The results were similar when mortality events prior to 1 year were censored.
Figure 1.
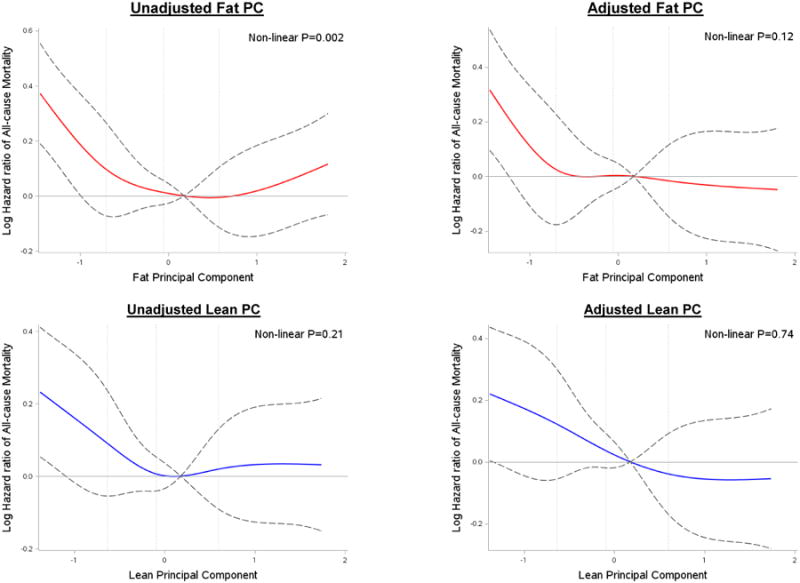
DEXA-derived tissue composition and risk of all-cause mortality. Unadjusted and fully adjusted association between fat /lean principal components and all-cause mortality. Vertical lines represent 25, 50 and 75th percentile cutpoints for PCs.
Table 3.
Multivariable-adjusted Cox regression models for mortality as a function of DEXA-derived tissue composition.
| All-cause mortality (765/993)* | Cardiovascular mortality (282/993)* | Non-cardiovascular mortality (483/993)* | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Fat and Lean PC | ||||||
| Fat PC Quartile 1 | 1.24 (1.04, 1.48) | 0.02 | 1.26 (0.94, 1.70) | 0.12 | 1.23 (0.98, 1.54) | 0.08 |
| 198/241* | 72/241* | 126/241* | ||||
| Fat PC Quartile 4 | 0.97 (0.81, 1.16) | 0.70 | 0.94 (0.70, 1.27) | 0.71 | 0.97 (0.77, 1.22) | 0.82 |
| 181/239* | 67/239* | 114/239* | ||||
| Lean PC | 0.91 (0.84, 0.98) | 0.01 | 0.84 (0.74, 0.95) | 0.005 | 0.95 (0.86, 1.05) | 0.29 |
Model was adjusted for age, sex, race, education, income, smoking, physical activity, eGFRcys, hypertension, diabetes, cardiovascular disease. Fat PC was modeled in quartiles, with the middle two quartiles (25th-75th percentile) as the reference category, given non-linear relationship in restricted cubic splines. Lean PC was modeled continuously based on the linear shape of the relationship in restricted cubic splines.
indicates number of deaths per total number of participants.
Table 4.
Multivariable-adjusted Cox regression models for mortality as a function of DEXA-derived tissue composition using raw DEXA components.
| All-cause mortality (765/993)* | Cardiovascular mortality (282/993)* | Non-cardiovascular mortality (483/993)* | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Total fat mass (kg/m2) | 0.97 (0.95, 0.997) | 0.02 | 0.96 (0.92, 0.998) | 0.04 | 0.98 (0.95, 1.01) | 0.20 |
| Trunk fat mass (kg/m2) | 0.95 (0.91, 0.99) | 0.02 | 0.93 (0.86, 0.996) | 0.04 | 0.96 (0.91, 1.01) | 0.13 |
| Total lean mass (kg/m2) | 0.98 (0.93, 1.02) | 0.26 | 0.95 (0.89, 1.02) | 0.15 | 0.99 (0.93, 1.05) | 0.72 |
| Appendicular lean mass (kg/m2) | 0.89 (0.81, 0.97) | 0.009 | 0.81 (0.70, 0.93) | 0.003 | 0.94 (0.84, 1.05) | 0.27 |
Models were adjusted for age, sex, race, education, income, smoking, physical activity, eGFRcys, hypertension, diabetes, cardiovascular disease and total fat mass (for lean tissue measures) or total lean mass (for fat tissue measures). Total fat mass, trunk fat mass, lean mass and appendicular lean mass were modeled continuously based on the linear shape of the relationship in restricted cubic splines. Hazard ratios per 1 kg/m2 of DEXA-derived tissue composition metrics are reported
indicates number of deaths per total number of participants.
Discussion
The relationship between fat and lean tissue distribution with cause-specific mortality in aging populations is controversial. We demonstrate that greater lean tissue had a protective association with all-cause and cardiovascular mortality. The lowest quartile of fat (as quantified by fat PC) was associated with greater all-cause mortality, while the upper quartile of fat was not associated with outcomes. These results underscore the complex relationship between DEXA-based lean and fat tissue and all-cause and cardiovascular outcomes in the elderly, highlighting the importance of lean tissue in cardiovascular risk with aging.
In an aging population, identifying clinical metrics that distinguish individuals at especially high risk for cardiovascular (and non-cardiovascular) events is critical to target dietary and exercise-based preventative efforts. Most research in this area has been focused on aggregate measures, such as BMI. In a meta-analysis of 2.88 million adults, Flegal and colleagues suggested a complex relationship between adiposity and survival: overweight status (BMI 25 to <30 kg/m2) was associated with improved survival, while grade 2 and 3 obesity (BMI 35+ kg/m2) were associated with poorer survival[1]. However, in the subgroup of individuals age ≥ 65 years, overweight status remained protective, but obesity was not associated with an increased mortality hazard[1], a finding that has been replicated in other studies[4, 23, 24]. On the other hand, in a separate pooled study of 1.46 million Caucasian adults 19-84 years of age, among individuals aged over 60 years who never smoked, the investigators observed an increasing mortality risk with greater BMI, starting in an overweight BMI range (>27.5 kg/m2)[25]. In addition, despite the well-known impact of visceral fat on cardiometabolic risk, studies investigating the prognostic relevance of visceral fat distributions in the elderly (using waist circumference) have been variable and potentially sex- and race-specific[26-30]. Less debate ensues at the other end of the spectrum (low adiposity is related to poorer survival[7]), where residual confounding by smoking, under-recognized chronic illness, potential metabolic reserves in adipose tissue have been advanced to explain the apparent protection from increased BMI in the elderly. Nevertheless, conflicting conclusions between overall or regional adiposity and outcome have fueled the need for more specific measures of tissue composition to define the physiology and epidemiology of aging-related alterations.
The advent of DEXA imaging in large epidemiologic aging cohorts has afforded an opportunity to address this issue directly using defined measures of body composition and the potential to address low lean mass (one aspect of “sarcopenia”), associated with poor prognosis in the elderly. In a recent study from the National Health and Nutrition Examination Survey (NHANES) examining 6,451 middle-aged adults with DEXA (average age 57.6 years, BMI 30 kg/m2), Srikanthan and colleagues found that cardiovascular mortality was lower with greater appendicular lean mass, trunk fat mass, and BMI[9]. Similar to our study, highest overall event rates were observed in the group with a combined low lean/low fat mass (the group with the lowest BMI); the high muscle/low fat group had the lowest event rates. Although the investigators found significant heterogeneity in event-free survival among different lean/fat groups, high versus low appendicular lean mass appeared to define differential long-term survival (even in high/low fat groups).
Our study extends these results into an older population (average age in CHS sample: 76.2 years), demonstrating that decreased lean tissue by DEXA is associated with poorer cardiovascular and all-cause mortality. In addition, similar to the younger cohort in NHANES, lower adiposity was associated with increased mortality. However, the highest quartile of fat— measured as a composite PC—was not significantly associated with outcome. Of note, in a recent study of nearly 50000 individuals (mean age 63.5 years) referred for clinical DEXA scans, Padwal and colleagues found that the highest quintile of body fat percentage (adjusted for BMI and clinical risk factors) was associated with mortality, suggesting that fat composition may explain obesity-related risk[8]. While the authors suggest that inclusion of body fat and BMI simultaneously in prognostic models inherently adjusts for lean mass (as BMI may reflect lean mass in this circumstance), our study uses whole-body DEXA with statistical methods (PCA) to define separate, independent axes of body composition to directly address prognostic importance of separate tissue depots. This PCA based approach (traditionally utilized as a method of variable reduction and unsupervised machine learning in large “-omics” datasets) provides an unbiased view of body composition metrics that capture (1) the interrelationship between different regional fat tissue and lean tissues in a single value for regression analyses; (2) limits the impact of collinearity, a limitation in assessing the prognostic importance of body composition.; (3) allows for simultaneous adjustment for distinct “lean” and “fat” tissue distribution.
An important finding in this study is the impact of decreased lean mass on outcomes in the elderly. Although the concept that “sarcopenia”—a decrease in muscle mass, quality, and function—is a major contributor to outcomes in the elderly is well established[31], thresholds for defining sarcopenia across sex and race and in the presence of comorbid illness remain open for debate. Nevertheless, decreased DEXA-derived appendicular lean mass[32-34] may define subsets of elderly individuals at high risk, and responds to dietary and exercise-based interventions. Importantly, our study uses careful DEXA-derived metrics (both PCA-derived composite scores of lean mass as well as previously defined measures of appendicular mass) to further define a role for sarcopenia in cardiovascular prognosis in the elderly. Mechanistically, sarcopenia has been recently linked to several key aspects of unhealthy aging that may worsen cardiovascular (and overall) prognosis, including decreased cardiorespiratory fitness[35], insulin resistance, pro-atherogenic dyslipidemia[36], systemic inflammation and coronary artery calcification, independent of sarcopenia-related insulin resistance[37]. Given the impact of exercise training on sarcopenia[38], in the context of prior literature, efforts in the elderly to improve lean muscle mass may be useful to prevent cardiovascular disease and (2) frailty associated with a low BMI may be primarily a function of reduced lean tissue mass. Indeed, future investigations that unite functional (e.g., gait speed), biochemical measures (e.g., metabolic or epigenetic), and anatomic measures of muscle quality (either via DEXA or more advanced imaging methods) will be instrumental in defining early non-invasive markers of muscle dysfunction and their impact on healthy aging.
Key limitations to all epidemiologic studies of adiposity and outcomes are residual confounding and reverse causation[39]: for example, a lower weight (or weight loss) may be related to underlying prevalent disease that is under-recognized, misclassified, or may not be amenable to standard regression adjustments (e.g., smoking). We attempted to control for these effects by excluding participants with a weight loss of 5% or greater and performed additional sensitivity analyses excluding prevalent chronic illnesses and smoking to reduce bias from preexisting chronic diseases and confounding by smoking. Of the 1335 individuals included in our analytic cohort, 530 (nearly 40%) had a chronic medical condition (COPD, cancer, liver, renal or cardiac disease), which is not unexpected with an aging cohort. Certainly, restriction of our analyses to CHS participants without any prior comorbidities would certainly address some bias from residual confounding; however, even with the inclusion of participants with comorbid illnesses, we did not observe an “obesity paradox” as traditionally defined.
Strengths of our study include the prospective evaluation of an elderly cohort alongside comprehensive phenotyping of body composition and clinical characteristics and high event rate. We restricted our analysis to individuals with DEXA measures. First, while DEXA is an accessible measure of body composition, it quantifies body composition from two-dimensional (2-D) images unlike computed tomography or magnetic resonance imaging (not available to us) which capture 3-D imaging data. Furthermore, while there were no systematic differences in age, sex, weight or BMI, those with missing DEXA had a lower proportion of black race, lower education and income, lower smoking rates, lower activity and a higher prevalence of cardiometabolic disease (Supplemental Table S3). Finally, we chose to model fat PC in quartiles (with the mid-50% of observations as referent) to estimate association with outcome given complex associations between mortality and fat in spline models (Figure 1), which may have led to a type 2 error (inability to observe significant associations with fat and mortality risk) based on cutpoints or reference. However, our spline models suggested that fat was associated with significant mortality risk only in the low fat regime. While further studies in large aging populations may further discern associations with fat, it is clear that decreased lean mass is linked to greater cardiovascular and all-cause mortality.
In conclusion, in this large cohort of community-dwelling elderly Americans, we found that greater lean tissue mass was associated with improved cardiovascular and overall prognosis. In addition, while the lowest levels of fat were linked to adverse prognosis, higher levels of adiposity showed no significant evidence of protection. Collectively, these results suggest prevention efforts in the elderly frail may be best targeted toward nutritional and exercise-based interventions targeting improvements in lean muscle mass.
Supplementary Material
A complex relationship between tissue composition and outcomes exists, apart from aggregate measures of obesity.
The lowest quartile of fat tissue (by DEXA) was associated with greater all-cause mortality, while the upper quartile of fat was not associated with outcomes.
Greater lean tissue had a protective association with all-cause and cardiovascular mortality.
Prevention efforts in the elderly frail may be best targeted toward nutritional and exercise-based interventions targeting improvements in lean muscle mass.
Acknowledgments
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: Dr. Shah is funded by grants from the National Institutes of Health (K23HL127099) and the American Heart Association (16SFRN31740000). There are no relationships with industry.
Footnotes
Disclosures: Dr. Murthy has minor stockholdings in General Electric.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Aferdita Spahillari, Email: aspahill@bidmc.harvard.edu.
Kenneth Mukamal, Email: kmukamal@bidmc.harvard.edu.
Christopher DeFilippi, Email: cdefilip@medicine.umaryland.edu.
Jorge R Kizer, Email: jorge.kizer@einstein.yu.edu.
John S. Gottdiener, Email: jgottdie@medicine.umaryland.edu.
Luc Djoussé, Email: ldjousse@partners.org.
Mary F. Lyles, Email: mlyles@wakehealth.edu.
Traci M. Bartz, Email: bartzt@uw.edu.
References
- 1.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 3.Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc. 2005;53:2112–8. doi: 10.1111/j.1532-5415.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 4.Auyeung TW, Lee JS, Leung J, Kwok T, Leung PC, Woo J. Survival in older men may benefit from being slightly overweight and centrally obese--a 5-year follow-up study in 4,000 older adults using DXA. J Gerontol A Biol Sci Med Sci. 2010;65:99–104. doi: 10.1093/gerona/glp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, et al. Skeletal muscle and mortality results from the InCHIANTI Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64:377–84. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CG, Boyko EJ, Nielson CM, Stefanick ML, Bauer DC, Hoffman AR, et al. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc. 2011;59:233–40. doi: 10.1111/j.1532-5415.2010.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rolland Y, Gallini A, Cristini C, Schott AM, Blain H, Beauchet O, et al. Body-composition predictors of mortality in women aged >/= 75 y: data from a large population-based cohort study with a 17-y follow-up. Am J Clin Nutr. 2014;100:1352–60. doi: 10.3945/ajcn.114.086728. [DOI] [PubMed] [Google Scholar]
- 8.Padwal R, Leslie WD, Lix LM, Majumdar SR. Relationship Among Body Fat Percentage, Body Mass Index, and All-Cause Mortality: A Cohort Study. Ann Intern Med. 2016;164:532–41. doi: 10.7326/M15-1181. [DOI] [PubMed] [Google Scholar]
- 9.Srikanthan P, Horwich TB, Tseng CH. Relation of Muscle Mass and Fat Mass to Cardiovascular Disease Mortality. Am J Cardiol. 2016;117:1355–60. doi: 10.1016/j.amjcard.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Allison DB, Zhu SK, Plankey M, Faith MS, Heo M. Differential associations of body mass index and adiposity with all-cause mortality among men in the first and second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) follow-up studies. Int J Obes Relat Metab Disord. 2002;26:410–6. doi: 10.1038/sj.ijo.0801925. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 12.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–7. doi: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 14.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 15.Kern LM, Powe NR, Levine MA, Fitzpatrick AL, Harris TB, Robbins J, et al. Association between screening for osteoporosis and the incidence of hip fracture. Ann Intern Med. 2005;142:173–81. doi: 10.7326/0003-4819-142-3-200502010-00007. [DOI] [PubMed] [Google Scholar]
- 16.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 17.Mannino DM, Davis KJ, Kiri VA. Chronic obstructive pulmonary disease and hospitalizations for pneumonia in a US cohort. Respir Med. 2009;103:224–9. doi: 10.1016/j.rmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 19.Fang J, Austin PC, Tu JV. Test for linearity between continuous confounder and binary outcome first, run a multivariate regression analysis second. In: Institute S, editor. Proceedings of the SAS Global Forum 2009 Conference. Cary, NC: 2009. Paper 252. [Google Scholar]
- 20.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54:201–8. doi: 10.1016/s0169-2607(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 21.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 22.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging. 2014;7:1221–35. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. Jama. 2005;293:1861–7. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 24.Bender R, Jockel KH, Trautner C, Spraul M, Berger M. Effect of age on excess mortality in obesity. JAMA. 1999;281:1498–504. doi: 10.1001/jama.281.16.1498. [DOI] [PubMed] [Google Scholar]
- 25.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han SS, Kim KW, Kim KI, Na KY, Chae DW, Kim S, et al. Lean mass index: a better predictor of mortality than body mass index in elderly Asians. J Am Geriatr Soc. 2010;58:312–7. doi: 10.1111/j.1532-5415.2009.02672.x. [DOI] [PubMed] [Google Scholar]
- 27.Lisko I, Tiainen K, Stenholm S, Luukkaala T, Hurme M, Lehtimaki T, et al. Are body mass index, waist circumference and waist-to-hip ratio associated with leptin in 90-year-old people? European journal of clinical nutrition. 2013;67:420–2. doi: 10.1038/ejcn.2013.39. [DOI] [PubMed] [Google Scholar]
- 28.Srikanthan P, Seeman TE, Karlamangla AS. Waist-hip-ratio as a predictor of all-cause mortality in high-functioning older adults. Ann Epidemiol. 2009;19:724–31. doi: 10.1016/j.annepidem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr. 2007;86:1339–46. doi: 10.1093/ajcn/86.5.1339. [DOI] [PubMed] [Google Scholar]
- 30.Hays JC, Keller HH, Ostbye T. The effects of nutrition-related factors on four-year mortality among a biracial sample of community-dwelling elders in the North Carolina piedmont. Journal of nutrition for the elderly. 2005;25:41–67. doi: 10.1300/j052v25n02_04. [DOI] [PubMed] [Google Scholar]
- 31.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikolov J, Spira D, Aleksandrova K, Otten L, Meyer A, Demuth I, et al. Adherence to a Mediterranean-Style Diet and Appendicular Lean Mass in Community-Dwelling Older People: Results From the Berlin Aging Study II. The journals of gerontology Series A, Biological sciences and medical sciences. 2015 doi: 10.1093/gerona/glv218. [DOI] [PubMed] [Google Scholar]
- 33.Meyer A, Salewsky B, Spira D, Steinhagen-Thiessen E, Norman K, Demuth I. Leukocyte telomere length is related to appendicular lean mass: cross-sectional data from the Berlin Aging Study II (BASE-II) Am J Clin Nutr. 2016;103:178–83. doi: 10.3945/ajcn.115.116806. [DOI] [PubMed] [Google Scholar]
- 34.Eibich P, Buchmann N, Kroh M, Wagner GG, Steinhagen-Thiessen E, Demuth I, et al. Exercise at Different Ages and Appendicular Lean Mass and Strength in Later Life: Results From the Berlin Aging Study II. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71:515–20. doi: 10.1093/gerona/glv171. [DOI] [PubMed] [Google Scholar]
- 35.Kim TN, Park MS, Kim YJ, Lee EJ, Kim MK, Kim JM, et al. Association of low muscle mass and combined low muscle mass and visceral obesity with low cardiorespiratory fitness. PLoS One. 2014;9:e100118. doi: 10.1371/journal.pone.0100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010;5:e10805. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ko BJ, Chang Y, Jung HS, Yun KE, Kim CW, Park HS, et al. Relationship Between Low Relative Muscle Mass and Coronary Artery Calcification in Healthy Adults. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.116.307156. [DOI] [PubMed] [Google Scholar]
- 38.Topcu Y, Tufan F, Karan MA. Resistance training might have improved insulin resistance by attenuating sarcopenia. Clinical interventions in aging. 2015;10:1935–6. doi: 10.2147/CIA.S98891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawlor DA, Hart CL, Hole DJ, Davey Smith G. Reverse causality and confounding and the associations of overweight and obesity with mortality. Obesity (Silver Spring) 2006;14:2294–304. doi: 10.1038/oby.2006.269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


