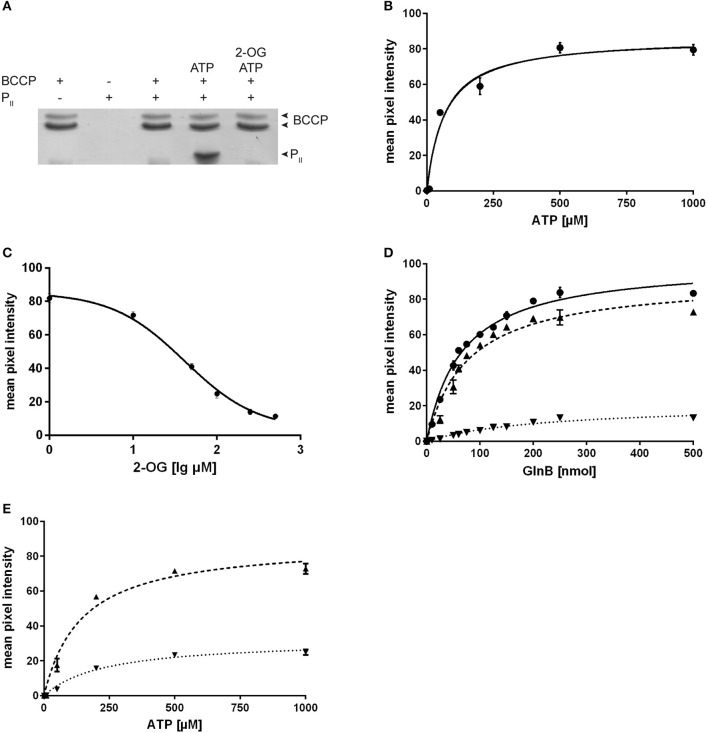
Figure 2.
(A) Pull down experiments of Synechocystis BCCP and GlnB using BCCP as bait. Experiments were performed using 0.5 mM Mg2+, 0.5 mM ATP and 1 mM 2-OG where indicated. (B) ATP titration of the BCCP-GlnB interaction while the amount of GlnB used was held constant and a calculated EC50 value of 68.0 μM (SE 13.2 μM) for ATP. The maximum binding was calculated to be 86.6 mpi (SE 3.8 mpi). (C) Influence of 2-OG on the BCCP-GlnB interaction. The IC50 of 2-OG is 41.3 μM (SE 1.7 μM). (D) Increasing amounts of GlnB and its S49 variants were used with BCCP as bait with 0.5 mM Mg2+ and 0.5 mM ATP. The EC50 is 65.9 nmol (SE 4.2 nmol) for wild type (circles), 77.0 nmol (SE 8.6 nmol) for S49C (triangles) and 224 nmol (SE 32.1 nmol) for S49D (inverted triangles). Maximum binding was calculated to be 100.7 mpi (SE 2.2 mpi) for the wild type protein, 91.4 mpi (SE 3.7 mpi) for the S49C variant and 21.0 mpi (SE 1.6 mpi) for the S49D variant. (E) ATP titration of the BCCP-GlnB S49C and S49D variant (as in B). The calculated ATP EC50 value for S49C variant is 143 μM (SE 24 μM) and 231 μM (SE 36 μM) for the S49D variant. Maximum binding was calculated to be 88.2 mpi (SE 3.8 mpi) for the S49C variant and 32.2 mpi (SE 1.7 mpi) for the S49D variant.