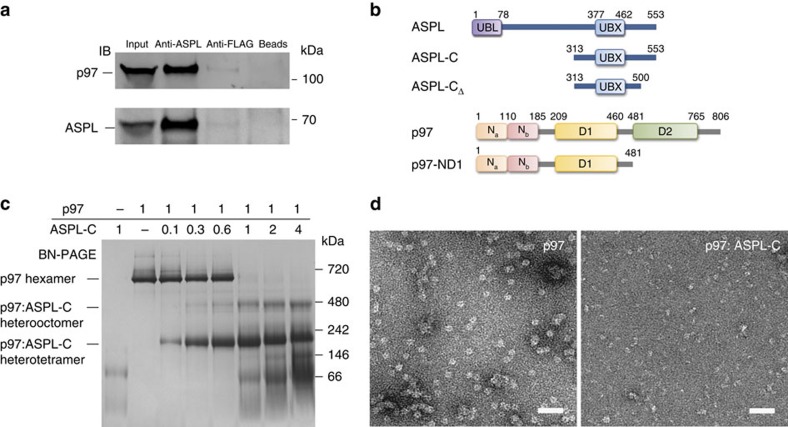
Figure 2. ASPL-C converts p97 hexamers into stable p97:ASPL-C heterooligomers.
(a) Co-immunoprecipitation of a p97:ASPL protein complex from human brain homogenate using an anti-ASPL antibody. Anti-FLAG antibody and beads alone were used as negative controls. (b) Schematic representation of fragments and full-length ASPL and p97. Conserved protein domains are depicted: ubiquitin-like domain (UBL); ubiquitin regulatory-X domain (UBX); N-terminal protein binding domains (Na and Nb); ATPase domains (D1 and D2). (c) Blue-native gel stained with Coomassie Brilliant Blue, demonstrating the remodelling of p97 hexamers by ASPL-C in a concentration-dependent manner. p97 (10 μg) and ASPL-C (0.3, 0.6, 1.8, 3, 6 and 12 μg) were briefly mixed and incubated on ice for 5 min; then protein complexes were analysed by BN-PAGE. A 1:1 molar ratio of p97 monomers and ASPL-C was sufficient to promote the formation of p97:ASPL-C heterooligomers. (d) Negatively stained electron micrographs of purified p97 in the presence and absence of ASPL-C; scale bar 50 nm.