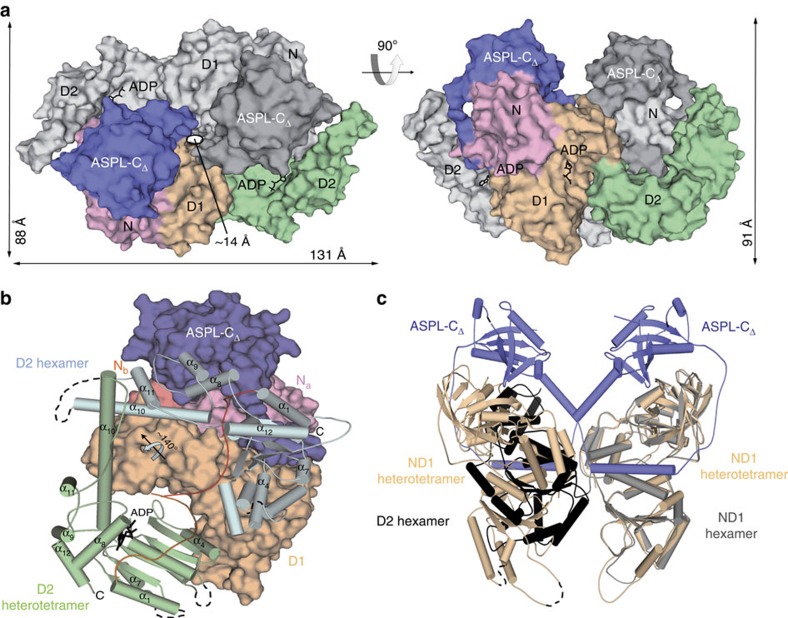
Figure 4. Remodelling of p97 hexamers by ASPL-CΔ induces a rearrangement of the distal ATPase domain D2.
(a) Surface representation of the heterotetrameric p97:ASPL-CΔ protein complex in two orthogonal orientations. One heterodimeric unit is displayed in shades of grey, the other shows ASPL-C in blue, p97-N in pink, p97-D1 in wheat colour and p97-D2 in green. (b) Structural comparison of the orientation of the D2 domain observed in p97 hexamers and p97:ASPL-CΔ heterotetramers. The N and D1 domains in the p97:ASPL-CΔ heterodimeric unit are shown in surface representation in colours as in a, the D1-D2 linker (orange) and the D2 domain (green) are shown in cartoon representation. The relative orientation of the D2 domain in p97 hexamers is shown in light blue with the D1-D2 linker in red. (c) Structural comparison reveals the potential cause for the reorientation of the D2 domain in p97. Superposition of a single protomer taken from the p97 hexamer (N and D1 in grey, D2 in black) onto the p97-ND1:ASPL-C heterotetramer (p97-ND1 in wheat colour, ASPL-C in blue) demonstrates a steric interference between the D2 domain and a p97-ND1:ASPL-C heterodimeric unit.