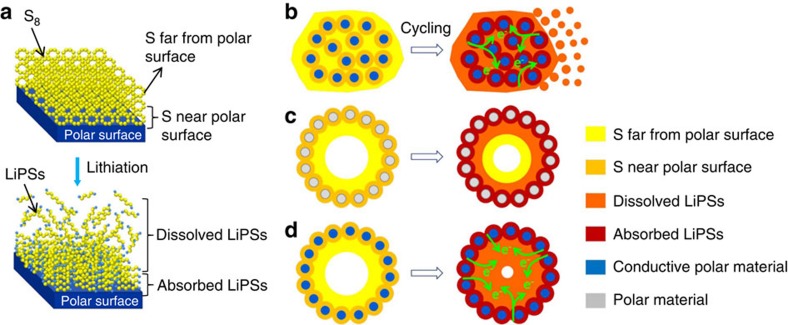
Figure 1. Schematic illustration of the adsorption limitation of LiPSs for polar hosts.
(a) LiPSs can be chemically adsorbed only when they are close enough to the polar surface, LiPSs far from the polar surface cannot be effectively anchored during the cycling. (b) Conductive polar nanoparticles can chemically adsorb LiPSs near their surfaces. When the sulfur content of the composite exceeds the limit, the extra LiPSs would dissolve into the organic electrolyte. (c) The hollow polar structure can bond with LiPSs near the surface, and effectively restrict the diffusion of the inner LiPSs. However, the low conductivity of the host hinders high sulfur utilization. (d) The conductive polar hollow structure inherits both advantages of b,c.