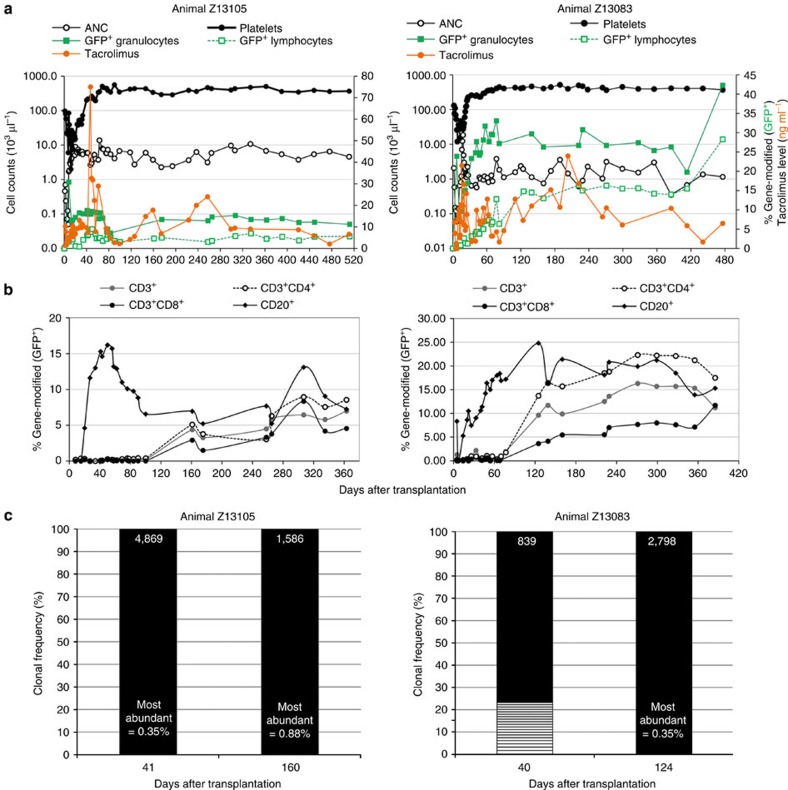
Figure 4. Sustainable haematopoiesis and engraftment of LV gene-modified CD34+ cells in vivo in NHPs following point-of-care manufacturing.
Two animals (Z13105 and Z13083) received autologous, LV gene-modified CD34+ cells produced under semi-automated conditions following myeloablative TBI. (a) Graphs depict haematopoietic recovery by ANCs (open black circles○) and platelet counts (closed black circles) on the primary y axis, engraftment of gene-modified PB granulocytes (closed green squares) and lymphocytes (open green squares) and measured tacrolimus levels in serum (closed orange circles) on the secondary y axis as a function of time after transplantation (x axis). (b) Percent of gene-modified (eGFP+) lymphocytes expressing CD3, and the percentage of eGFP+/CD3+ cells expressing CD4 and/or CD8 observed in PB (y axis) over time after transplant (x axis). (c) Highly polyclonal engraftment of LV gene-modified NHP CD34+ haematopoietic cells following point-of-care production and transplant. Bar graphs represent the clonal diversity of LV gene-modified PB leukocytes collected at day +40 after transplant into autologous recipients as determined by genomic locus of integration. Clonal integration site sequences constituting ≥1% of all sequences captured are indicated by boxes in ascending order of frequency. Coloured boxes indicate clones identified across time points. Total number of clones identified is listed atop each bar.