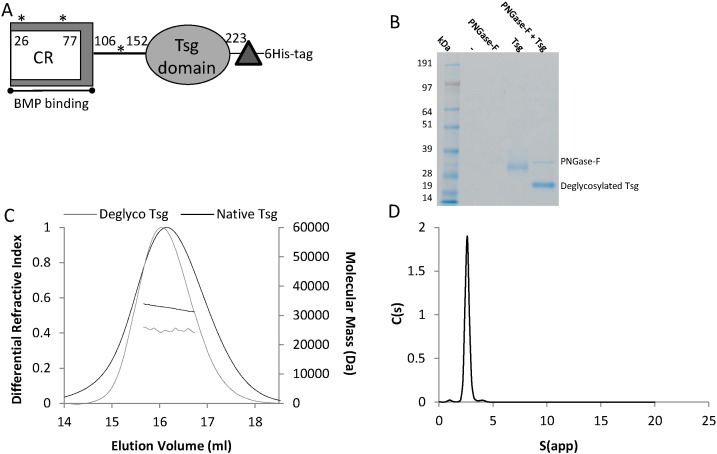
Fig. 1.
Glycosylation and oligomeric state of Tsg. (A) Schematic diagram of Tsg. Tsg has previously been predicted to be composed of two domains, the first having homology to the cysteine-rich (CR) von Willebrand C-type domains in chordin and binds to BMPs [14] whereas the second (Tsg-specific domain of as yet unknown function) also contacts a high number of cysteine residues but has no known homologues. The domains are separated by a putative hinge region and both domains appear to be required for chordin binding [38]. The construct contains a 6-His-Tag for purification and a thrombin cleavage site for tag removal (triangle). Potential N-linked glycosylation sites indicated by asterisks. (B) Coomassie stained SDS-PAGE gel showing that purified Tsg can be deglycosylated under non-denaturing conditions. (C) MALS profile of native and deglycosylated Tsg. Refractive index and calculated molecular weight against elution volume from the size exclusion column (ml) showing the shift in elution volume and size following deglycosylation. Native Tsg (black line) has a molecular mass of 32.3 kDa (± 0.13%), ~ 30% larger than deglycosylated Tsg (grey line) (25.0 kDa (± 0.5%)) experimental errors from polydispersity. In both states the mass of Tsg is consistent with a monomer in solution (theoretical mass based on peptide sequence is 23.8 kDa). (D) Velocity AUC data for native Tsg showing a single species with mass ~ 36.7 kDa, S20W of 2.57, hydrodynamic radius of 3.44 nm and frictional ratio of 1.27.