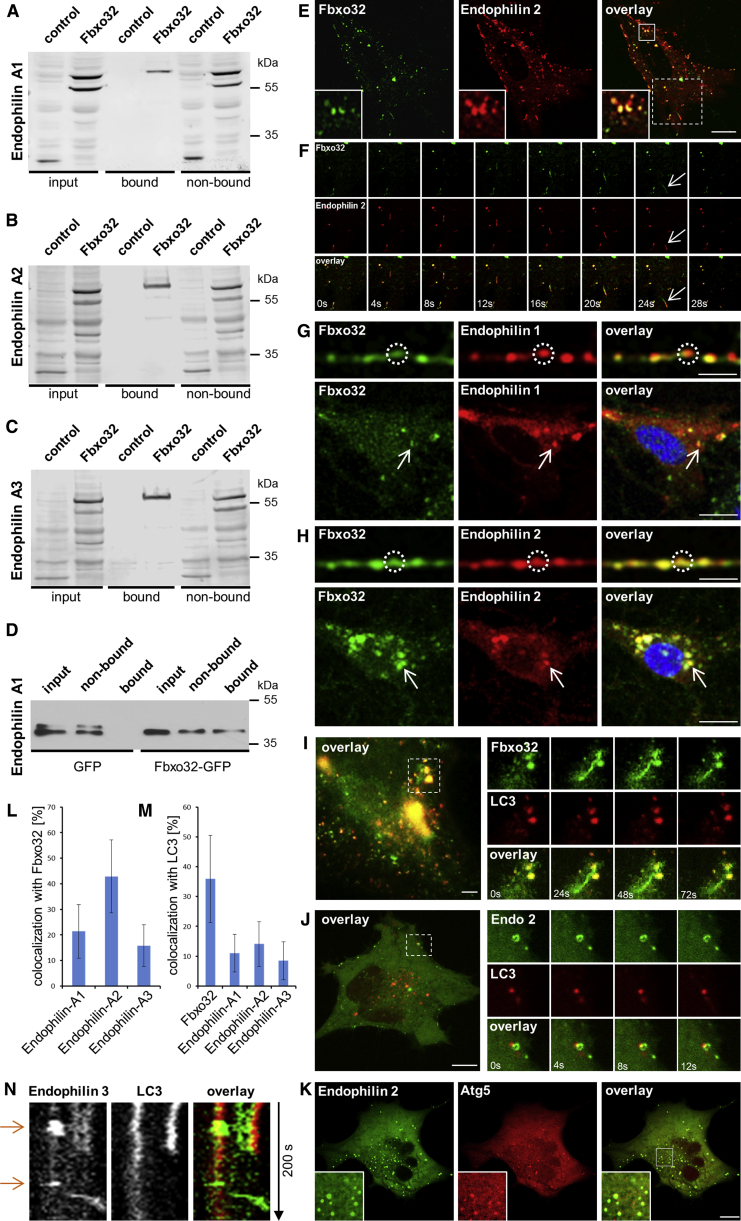
Figure 5.
FBXO32 and Endophilin-A Interact and Colocalize Transiently on Organelles and Tubular Structures
(A–C) Endophilin-1 (A), 2 (B), and 3 (C) interact with FBXO32. Anti-GFP immunoprecipitation from HeLa cell lysate probed with anti-RFP antibody is shown (three experiments).
(D) FBXO32-GFP-coupled beads pull-down purified endophilin-1.
(E) MEF overexpressing FBXO32-EGFP (left) and endophilin A2-RFP (middle); magnified in the inset. Note that FBXO32 and endophilin-2 colocalize on organellar and tubular structures. The scale bar represents 7 μm.
(F) Time-lapse images from (E) showing dynamic tubular structure with FBXO32 and endophilin-2. Note FBXO32’s association with membrane tubules independently of endophilin-2. Inset size is 4 μm.
(G and H) Primary hippocampal neurons expressing FBXO32-EGFP and endophilin-1-mRFP (G) or endophilin-2-mRFP (H), transfected by electroporation. (Up) Neuronal processes are shown (scale bar 3 μm). (Bottom) Neuronal cell bodies are shown (scale bar 7 μm).
(I) FBXO32-EGFP colocalization with LC3-mRFP in starved MEF. A representative cell shows the overlay between FBXO32-EGFP and LC3-mRFP (imaged by near-TIRF microscopy). Panels on the right show dynamic FBXO32-labeled tubule. Movie S4 shows trafficking of FBXO32 and LC3 in the same cell. The scale bar represents 3 μm.
(J) HeLa cell expressing LC3-EGFP (left) and endophilin-A2 protein (middle), starved for 2 or 3 hr. The scale bar represents 3 μm.
(K) Endophilin-2-EGFP transiently colocalized with ATG5-mCherry (early autophagosomal marker) in starved HeLa cell. Magnified is shown in the inset. The scale bar represents 5 μm.
(L and M) Quantification of colocalization of endophilins with FBXO32 (L) and LC3 (M). Random colocalization has been subtracted. Mean ± SD.
(N) Kymograph of image series (200 s) showing a transient colocalization of endophilin-3 (green) and LC3 (red) in a HeLa cell imaged by spinning-disk microscopy.