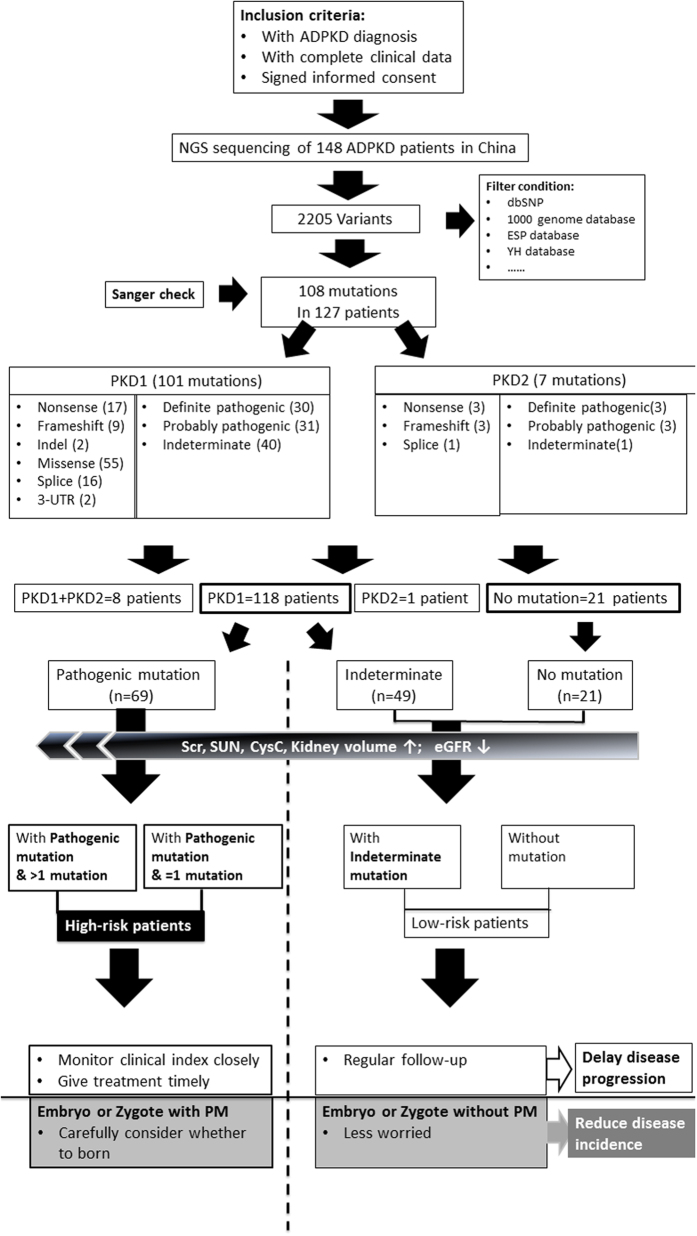
Figure 1. Flow diagram of genetic diagnosis (mutation detection and pathogenic prediction in PKD1/PKD2) based on the next-generation sequencing platform, and the clinical significance of genetic diagnosis for delaying progression and reducing the incidence of ADPKD: ① one hundred and forty eight patients diagnosed with ADPKD were enrolled, and their peripheral blood was subjected to next-generation sequencing.
After a comparison with databases, normal variations and artefact variants were filtered out, resulting to 108 mutations detected in 127 patients (85.8%). Of these 148 patients, one hundred and eighteen (79.7%, 118/148) harboured a mutation in PKD1, 1 (0.7%, 1/148) harboured a mutation in PKD2, 8 (5.4%, 8/148) harboured mutations in both PKD1 and PKD2, and 21 lacked PKD1/PKD2 mutations (14.2%, 21/148). The pathogenicity of mutations was predicted, and they were categorized into three types (definite pathogenic mutation, probable pathogenic mutation, and indeterminate mutation). Thus, the patients were divided into three groups: patients with pathogenic mutation, patients with indeterminate mutation, and mutation-free patients. The association of genotype/phenotype showed that patients with a pathogenic mutation had higher serum creatinine levels, higher serum urea nitrogen levels, higher cystatin c levels, larger kidney volumes, and lower eGFR levels than patients with indeterminate mutations or mutation-free patients. Based on these data, ADPKD patients were categorized into two groups: the high-risk group (with pathogenic mutations) and the low-risk group (with indeterminate mutation or no mutation). ② Including genetic diagnosis in clinical practice will likely reduce the economic cost of the disease and reduce monitoring patients destined to be symptom-free while proactively increasing preventive monitoring for patients at high risk for progressive renal disease to help delay the progression of ADPKD. In addition, genetic diagnoses of embryos or even zygotes for ADPKD patients who have a family plan may provide reasonable fertility recommendations to help decrease the incidence of ADPKD.