Figure 3.
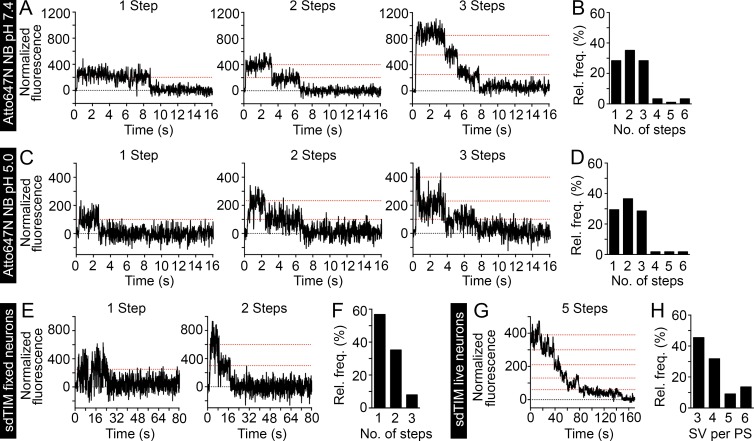
sdTIM enables the simultaneous detection of multiple SVs in live hippocampal nerve endings. Highly diluted Atto647N nanobodies were deposited on a glass surface, and the fluorescence emission was recorded over time. (A and B) At pH 7.4 (n = 90 Atto647N nanobodies), 28% of traces show one, 36% show two, and 28% show three steps. (C and D) At pH 5.0 (n = 106 Atto647N nanobodies), 29% of traces show one, 37% show two, and 29% show three steps. At both pHs, a small number of traces (<8%) showed more than three steps, as a consequence of more than one antibody being found within the same diffraction-limited spot. The graphs in B and D show the relative frequencies of the detected emission steps. To estimate the number of fluorophores per SV, hippocampal neurons expressing VAMP2–pHluorin were subjected to sdTIM, as described in Fig. 1, and fixed. Representative one- and two-step fluorescence time (s) traces obtained from fixed presynapses are shown (E) together with the relative frequency (%) of Atto647N emission steps after bleaching (F). Note that in the majority of cases, the SVs exhibited only one fluorescence step, with an average ratio of Atto647N fluorophores per nanobody of 1.5:1. (G) After sdTIM, a representative fluorescence-time trace from a live hippocampal neuron presynapse is shown. (H) Relative frequency of detected SVs in live presynaptic terminals. Dotted red lines in the graphs indicate the detected emission steps. n = 88 traces from 22 presynapses originating from five neurons.